Abstract
The 2019 outbreak of corona virus disease began from Wuhan (China), transforming into a leading pandemic, posing an immense threat to the global population. The WHO coined the term nCOVID-19 for the disease on 11th February, 2020 and the International Committee of Taxonomy of Viruses named it SARS-CoV-2, on account of its similarity with SARS-CoV-1 of 2003. The infection is associated with fever, cough, pneumonia, lung damage, and ARDS along with clinical implications of lung opacities. Brief understanding of the entry target of virus, i.e., ACE2 receptors has enabled numerous treatment options as discussed in this review. The manuscript provides a holistic picture of treatment options in COVID-19, such as non-specific anti-viral drugs, immunosuppressive agents, anti-inflammatory candidates, anti-HCV, nucleotide inhibitors, antibodies and anti-parasitic, RNA-dependent RNA polymerase inhibitors, anti-retroviral, vitamins and hormones, JAK inhibitors, and blood plasma therapy. The text targets to enlist the investigations conducted on all the above categories of drugs, with respect to the COVID-19 pandemic, to accelerate their significance in hindering the disease progression. The data collected primarily targets recently published articles and most recent records of clinical trials, focusing on the last 10-year database. The current review provides a comprehensive view on the critical need of finding a suitable treatment for the currently prevalent COVID-19 disease, and an opportunity for the researchers to investigate the varying possibilities to find and optimized treatment approach to mitigate and ameliorate the chaos created by the pandemic worldwide.
Keywords: Corona virus, Pandemic, SARS-CoV-2, ACE-2, Anti-viral drugs, Blood plasma therapy
Introduction: the origin of the pandemic
The human respiratory system is the primary target of severe acute respiratory syndrome corona virus-2 (SARS-CoV-2) pathogen, contributing to the occurrence of COVID-19 disease posing critical threats to the population (Rothan and Byrareddy 2020). The nCOVID-19 depicts novel (n) corona (CO) virus (VI) disease (D) and the numeral 19 depicts the year of its outbreak, i.e., 2019. Gradual progress in identification, diagnosis, clinical studies, and management of this virus became a major concern, and the first US case of corona virus led to a symptomatic analysis with mild symptoms at initial stages progressively deteriorating to pneumonia up to the 9th day of infection (Holshue et al. 2020). Various reports have demonstrated horrific images of corona virus pandemic, which has now taken over countries like Italy and America, where death toll has reached alarming levels. The onset of symptoms begins after 5.2 days of exposure to the virus (Li et al. 2020) and the fatality risks begin after about 14 days of symptom onset (Wang et al. 2020a, b, c, d). The fatality rate depends upon the immune system efficiency of the patient, thus greater in geriatrics (above 70 years), children (< 5 years) and other patients subjected to immune disorders (Wang et al. 2020a, b, c, d). Cough, fever, shortness of breath as well as tiredness are the initial symptoms at the beginning of the infection, progressively leading to haemoptysis, dyspnoea, sputum production, diarrhea, headache, and lymphopenia (Carlos et al. 2020). On inhalation of the virus mediated release of signaling proteins, called interferons, the condition of the patients was expected to improve, but instead it worsened against COVID-19 infection (Lei et al. 2020). Although, the virus is a novel form, but similarities in the symptoms with the previous betacoronavirus were observed (Huang et al. 2020). Corona virus infections are associated with systemic problems like cough, fever, fatigue, sputum production, hypoxemia, haemoptysis, dyspnoea, cardiac injury, diarrhea, lymphocytopenia, and respiratory disorders, as per patient analysis and clinical implications (Rothan and Byrareddy 2020). The gastrointestinal symptoms observed in infected patients made it necessary to carry out fecal and urine examination to eliminate transmission risks (Assiri et al. 2013). The novel corona virus is a new category (7th human corona virus) came to emergence in 2019, in Wuhan after previous outbreaks like human corona virus 229 E (alphacoronavirus) and 1960s human corona virus OC43 (betacoronavirus), SARS-CoV-1 (betacoronavirus) in 2003, 2004 HCoV-NL63 (alphacoronavirus), 2005 HCoV-HKU1 (betacoronavirus), and 2012 MERS-CoV (betacoronavirus) (Devaux et al. 2020). The progression of corona virus infection is rapid and continuous, despite the stringent measures employed to control the transmission. The phylogentic interpretations relate the novel corona virus to SARS-CoV-1 up to some extent (Zhou et al. 2020). Associated with the family coronaviridae in the order nidovirales, corona virus comprises of surface spikes (crown-shaped) on the outside (Adnan et al. 2020). The diameter of the virus ranges from 65 to 125 nm and the genetic material comprises of 26–32 kbs long sequence of ssRNA. The family of corona virus has four categories, namely, alpha, beta, gamma, and delta. Other than SARS-CoV (2002), and COVID-19, the other corona virus categories primarily affected animals only (Zhong et al. 2003). Another similarity between SARS-CoV and COVID-19 is that both their outbreaks emerged from China, Guangdong province associated with SARS-CoV and Wuhan province with COVID-19. Soon after the outbreak, the Chinese researchers named it as 2019 novel corona virus (2019-nCoV)/Wuhan corona virus, altered by International Committee of Taxonomy of Viruses (ICTV), who named it SARS-CoV-2 (because of its genetic similarity with SARS-CoV of 2003), and the disease was called the COVID-19 (Cui et al. 2019). The novel corona virus is the advanced form, rendering more disastrous effects than SARS-CoV (2003). The SARS outbreak spread across 26 countries, with 9% mortality rate, whereas, the infection spread across 212 countries, with 2.9 mortality rate, in the present novel corona virus pandemic (Adnan et al. 2020). Therefore, a possible reason for the enhanced transmission potential of novel corona virus is genetic recombination in S protein of RBD region of the virus (Adnan et al. 2020). Going back to the previous cases of corona virus outbreak across the globe, the 2003 SARS-CoV came to existence from Guangdong province (China), infecting patients with pneumonia and alveolar injury, progressing into acute respiratory distress syndrome (Adnan et al. 2020). After this outbreak in 2012, MERS-CoV took hold of Saudi Arabian population infecting about 2428 people, causing death of 838 individuals with 36% mortality rate, according to WHO reports, deteriorating human health from mild to severe conditions that also led to kidney failure and ARDS (Rahman and Sarkar 2019). The present day scenario revealed a similar condition emerged in 2019, with many cases of pneumonia with unknown etiology. Previously, the visits to seafood markets (where the animals might have been infected) were considered primary causes of this infection, but later on the transmission potential of the virus was discovered on account of its emergence in those people who had not been to the seafood market (Adnan et al. 2020). Thus, it is the viral transmission that can be due to close contact, coughing, and sneezing, via which the infected aerosols penetrate healthy individuals (Phan et al. 2020). The origin of an infection is the most essential aspect to study in order to formulate effective treatment policies. The raccoon dogs and palm civets were investigated upon, as they were thought to be responsible for SARS-CoV outbreak, but as investigation results only found palm civets to play secondary hosts in viral progression (Kan et al. 2005). Furthermore, the MERS-CoV investigators propounded the involvement of bats in the viral infection progression and transmission, as its presence in pipistrellus and perimyotis bats was studied (Huynh et al. 2012). More work and research should be done in this regard to facilitate development of sufficient measures to eradicate the progression of the infection. The WHO has issued surveillance draft (January, 2020), demonstrating that any individual from Wuhan, 2 weeks before the symptom appearance, is suspected to be infected (Elfiky 2020). Moreover, the laboratories regulating tests and investigations against these viral infections were also notified with interim guidance by the WHO. A “public health emergency of international concern” was announced by the WHO on January 30th, in order to spread global awareness about the outbreak and was declared a global pandemic on March 11th, 2020 (Chavez and Long 2020). The previously emerged viral outbreaks have granted adequate lessons regarding emergency measures to be taken during pandemic, protective equipment, role of emergency equipment, and necessary guidelines for the medical practitioners. The transmission of the virus is the major consideration in mitigating the effects of a pandemic. The SARS-C0V-2 exhibits person to person transmission via respiratory droplets (aerosols) (“CDC” 2020b). Eyes, nose, and mouth are susceptible to infection from contaminated surfaces or fomites (“CDC” 2020b). The transmission potential of the virus exceeds once the symptoms begin to show (“Coronavirus”). Critically infected patients exhibit greater viral shedding as compared to mild infectious patients, as per the few clinical results (Zou et al. 2020). For every COVID-19 infected patient, the reproduction number is > 2.2 and greater than 2 cases have maximum possibility to prevail in case the infected patient is not adequately isolated (Li et al. 2020). The severity of viral infection varies with different types of patients, where age and gender plays a critical role. The place of origin of the COVID-19 infection was found to affect patients with an average age of 59 years, out of which about 56% were males, as per the epidemiological studies (Li et al. 2020). The severity of the infection is categorized as per certain characteristics like PAO2/FiO2 greater than 300, respiration exceeding to more than 30 breaths per minute, dyspnea, presence of more than 50% lung infiltrates with 1 to 2 days, and 93% or more oxygen saturation are the significant parameters that contribute to severe COVID-19 infection (Zhang et al. 2020a) whereas, septic shock, multiple organ failure, and respiratory distress are associated with critical patients, where the fatality rate is very high, i.e., 49%. The increased death possibility greatly depends upon the age of the patients, evidently depicting increased mortality rate among geriatrics (> 80 years of age) (Chavez and Long 2020). Moreover, patients with deteriorated medical condition and health issues also face greater mortality rate than normal individuals, such as 10.5% death rate in patients with CVDs (Chavez and Long 2020). Furthermore, according to the global genome sequence reports, the ability of the virus to mutate is greater than any other pandemic or epidemic virus that has even come into existence (Islam et al. 2021a, b). The elevated infectivity and high mutation potential facilitates rapid progression to virus to multiple parts of the world, in a short period of time, causing a devastating increase in the death rate. Multiple factors affecting the mutation rate of SARS-CoV-2 comprise of cellular metabolism (reactive oxygen species, metabolites), genomic errors (polymerase proof reading, gain of functions mutations), proteomic errors (mutation in spoke protein), and environment-induced mutations (UV, X-ray) (Islam et al. 2021a, b). Numerous challenges have been reported in the development of vaccines and drugs in the current pandemic, as the virus might gain resistance towards the novel therapeutic agents and interventions, on account of mutations (Islam et al. 2021a, b). Thus, a clear understanding of the genetic variability might significantly aid in limiting the spread of virus and control the infection.
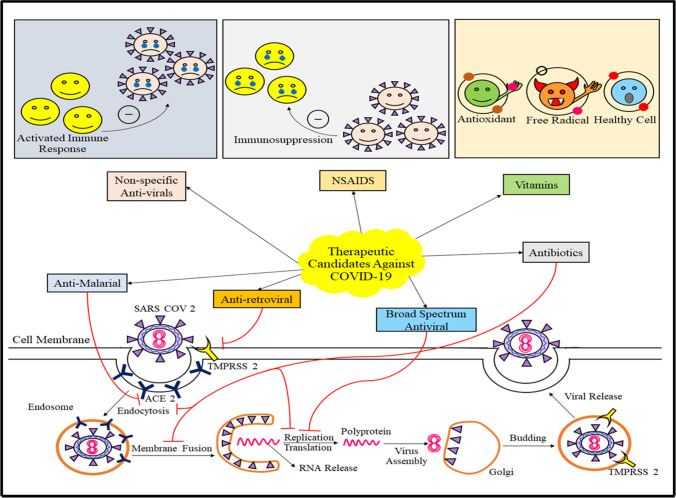
The authors entail the possible therapeutic approaches in the COVID-19, targeting the anti-malarial drugs, non-specific anti-inflammatory and immune system suppressing agents, anti-HCV, nucleotide inhibitors, repurposing agents, anti-parasitics and antibodies, non-specific antiviral drugs, RNA-dependent RNA polymerase inhibitors, antiretrovirals, vitamins and hormones, JAK inhibitors, synthetic virus particles, as well as blood plasma therapy, along with collection of clinical trials centered around these categories of drug candidates. The manuscript aims to provide an opportunity to the researchers to scrutinize the treatment possibilities to establish an effective and reliable treatment option to mitigate the COVID-19 pandemic.
Treatment approaches in COVID-19 infection
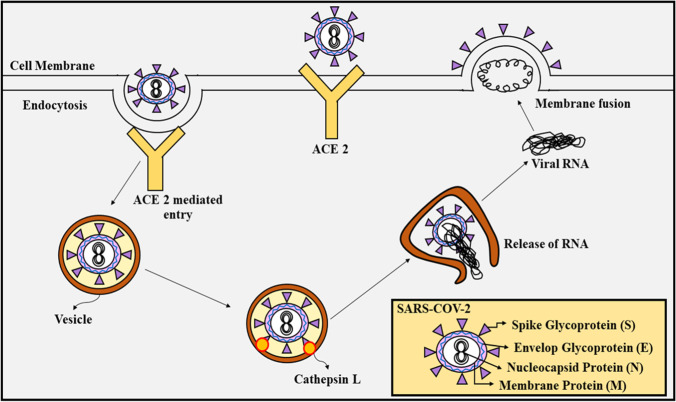
The treatment criteria of COVID-19 is linked to past out breaks of corona virus, along with the mechanism of entry of the virus into the host cell, via angiotensin-converting enzyme (ACE-2) and TMPRSS2, as shown in Fig. 1. The treatment requires radiographic evidence, infection information, possible risk factors, and cases without proper care at home (“WHO” 2020b). To determine whether the household environment is suitable to harbor such a patient or hospital premises is required, CDC has laid certain guidelines (“CDC” 2020d). Similarly, the patients requiring ICU admission also possess certain for admission as determined by “WHO” (“WHO” 2020b). The criteria involve tachypnea associated severe cases of pneumonia, critical respiratory imbalance, improper oxygenation, and respiratory rate of > 30 breaths/minute (“WHO” 2020b). In children, the criteria for the same involve cyanosis, respiratory imbalance, breastfeeding or drinking in capabilities, altered consciousness, tachypnea, tiredness, and seizures (“WHO” 2020b). The breathing rate varies with age of the children (doubtful of infection), like 60 or more breath/min in children (with age < 2 months), 50 or more breaths/min in children (with age 2 to 11 months), and 40 or more breaths/min in children (with age 1–5 years) (“WHO” 2020b). The public health authorities should conduct the treatments of the patients. A proper specialist should be appointed for diagnosis of the infection along with a pulmonologist to provide proper mechanical ventilation to the patient. Fluid balance and hemodynamic support should be significantly managed by critical care consultants. High flow nasal cannula (HFNC) is significantly recommended for patients with hypoxemic respiratory disturbance, instead of non-invasive positive pressure ventilation (NIPPV) (Alhazzani et al. 2020). Although, NIPPV may lead to significant improvements in patient health, yet it is not recommended as it enhances the aerosolization risk in patients (Alhazzani et al. 2020). As an alternative approach, patients can also be intubated, along with adequate airborne precautions along with ventilation with 4–8 mL/kg TV depending upon the body weight of the patient (“WHO” 2020c). ARDS-associated patients are subjected to more than 12 h of ventilation each day (“WHO” 2020c). Oxygenation can be worsened by excessive resuscitation using IV fluids, therefore it is not recommended. If sepsis is determined a one of the symptoms, then within 1 h of its determination, administration of empiric antibodies and a neuraminidase inhibitor is recommended by “WHO.” To mitigate shock in infected patients, buffered crystalloids are recommended instead of hypotonic crystalloids. Vasopressor agents, primarily norepinephrine is also recommended for shock (dose depends upon severity of infection) (“WHO” 2020c). Dobutamine is recommended to the infection that is accompanied with cardiac dysregulation (Alhazzani et al. 2020). In case of vasopressor, refractory shock and systemic steroids, like hydrocortisone (200 mg/day), are recommended (Alhazzani et al. 2020; “WHO” 2020c). In China, the combination of lopinavir and ritonavir (approved by the FDA for treatment of HIV infection) is being employed, along with interferon alpha (INF-γ) for COVID-19 therapy in infected patients (Harrison 2020). The infection cases accompanied with cardiac risks can be mitigated to some extent by using azithromycin and hydroxychloroquine combinations (“Clinical”). Moreover, antiviral agents, like remdesivir, are examined with potent in vitro action against the virus (Sheahan et al. 2020). Severe cases of infection are linked with diseased states like diabetes, CVDs, and hypertension (Huang et al. 2020). Special cases like HIV positive patients, if infected with COVID-19, should continue with their anti-retroviral treatment regimes, as per the guidelines given by the US Department of Health and Human Services (“Department”). In pregnant mothers, the data suggests the increase in their susceptibility towards COVID-19 infections. Exposure to the virus may lead to preterm birth, as investigated via certain studies (“CDC” 2020a). Appropriate guidelines suggesting adequate care to be taken of pregnant mothers have been issued by WHO (“WHO” 2020b), which also demonstrates that optimum delivery method to be chosen depending upon patient compliance and fetal condition. After delivery, in case the mother is at risk of COVID-19 infection, then the infant remains isolated from the mother until the risks are resolved, and the breast milk is provided by another caregiver (“CDC” 2020c). Table 1 enlists different drugs discussed in the manuscript, along with their categories, characteristics, and mechanism of action, with respect to their significance in the COVID-19 pandemic (Fig. 2).
Fig. 1.
Mechanism of entry of SARS-CoV-2 in host cell
Table 1.
Optimum drug categories, their candidates, and mechanism against COVID-19
| Category | Drug | Features | Action mechanism | Reference |
|---|---|---|---|---|
| Non-specific anti-virals | IL-2 | Cytokine signaling molecule | Elevation in number of CD8 + T cells, NK cells and CD4 + T cells | (“Chictr” 2020b) |
| Ig | Fc receptor activation blocker | Reduction in infection progression, dependent on antibody; increased endogenous Nabs | (Cao et al. 2020a; “Clinicaltrials” 2020e) | |
| INFβ-1a | Cytokine signaling molecule | Increased cytoplasmic enzymes; ameliorated mRNA translation and synthesis of proteins | (“Clinicaltrials” 2020o) | |
| INFβ-1b | Cytokine in INF-family | Increased cytoplasmic enzymes; ameliorated mRNA translation and synthesis of proteins | (Hung et al. 2020) | |
| CYNK-001 | Cryopreserved allogenic, NK cell therapy, derived from placenta | Elevated number of CD56 + /CD3- NK cells | (“Clinicaltrials” 2020l) | |
| Baloxavir marboxil | Cap snatcher blocker | Deceased level of endonuclease, dependent on viral cap | (Lou et al. 2021) | |
| Broad-spectrum anti-virals | Arbidol | Direct anti-viral, targeting host | Reduction in membrane haemagglutinin fusion | (“Smartpatients” 2020) |
| Remdesivir | Adenosine analog | Reduction in viral replication; curbed RNA polymerase | (Scavone et al. 2020; Wang et al. 2020c) | |
| Favipiravir | Blocker of RNA polymerase | Decreased RdRp | (“Clinicaltrials” 2020w; Furuta et al. 2017) | |
| Galidesivir | Adenosine analog | Reduced viral RNA polymerase | (“Clinicaltrials” 2020q) | |
| Anti-retrovirals | Azvudine | Nucleoside analog | Reduction in reverse transcriptase and viral replication | (“Chictr” 2020e) |
| Darunavir and cobicistat | Protease inhibitor | Reduction in Cytochrome P-450 CYP3A | (“Clinicaltrials” 2020b) | |
| Danoprevir | Protease inhibitor | Reduction in transcription and replication of viral particles | (Chen et al. 2020a, b) | |
| Lopinavir and ritonavir | Protease inhibitor | Ritonavir-mediated reduction in Cytochrome P450; curbed half life of lopinavir | (Cao et al. 2020a, b; Dorward and Gbinigie 2020) | |
| ASC09 | Protease inhibitor | Hindered proteolytic cleavage | (“Clinicaltrials” 2020f) | |
| Anti-malarials | Hydroxy-chloroquine | Anti-malarial | Decreases ACE-2 glycosylation; when coupled with remdesivir, decreases viral replication | (“Clinicaltrials” 2020m) |
| Antibiotics and anti-parasitics | Carrimycin | Polyether antibiotic | Prevents infection from fungi, yeast, gram positive bacteria and mycoplasma | (“Clinicaltrials” 2020v) |
| Ivermectin | Anti-parasitic | Decreased viral replication | (“Clinicaltrials” 2020r) | |
| Azithromycin | Macrolide antibiotic | Inhibits internalization into the host, during early infection stages | (“Clinicaltrials” 2020q; Tran et al. 2019) | |
| Suramin sodium | Treatment of trypanosomiasis and onchocerciasis | Decreased ACE2 glycosylation; curbed quinone reductase 2 | (“Chictr” 2020d) | |
| Doxycycline | Semi-synthetic tetracycline derivative | Reduced viral replication and IL-6 levels | (“Clinicaltrials” 2020v; Sargiacomo et al. 2020) | |
| Dihydroartemisinine and piperaquine | Viral Fc receptor activation blocker | Interaction between haem iron and peroxide bridge accounts for anti-viral action | (“Chictr” 2020a) | |
| Anti-inflammatory and immunosuppressive agents (non-specific) | Ibuprofen | NSAID | Reduction in COX and PGs | (“Clinicaltrials” 2020j; Cole and Frautschy 2010) |
| Corticosteroids | Immunomodulatory and anti-inflammatory | Curbed inflammation and regulation of immune system | (“Clinicaltrials” 2020a) | |
| Naproxen | NSAID | Retards COX activity | (“Clinicaltrials” 2020d) | |
| Thalidomide | Sedative and immunosuppressant | Reduced TNF-α and cell-surface adhesion molecules | (“Clinicaltrials” 2020c) | |
| Leflunomide | Immunosuppressant and DMARD | Suppressed dihydro-orotate dehydrogenase enzyme, tyrosine kinase enzyme; reduction in transcription factors in the cell | (“Clinicaltrials” 2020i) | |
| Colchicine | Antigout and anti-inflammatory | Retarded microtubule assembly; decreased chemotaxis and LTs; reduced activation of inflammasome | (“Clinicaltrials” 2020x) | |
| Vitamins | Vitamin C | Antioxidant potential | Mitigated respiratory failure during intubation; decreased inflammation; curbed accumulation of neutrophils in lungs | (“Clinicaltrials” 2020g; “Clinicaltrials” 2020s; “Clinicaltrials” 2020t) |
| Vitamin D | Immune system modulator | Decreased RAS and lung damage | (“Clinicaltrials” 2020h; “Clinicaltrials” 2020u; “Clinicaltrials” 2020y) | |
| Vitamin D3 | Immune system modulator | Decreased lung damage | (“Clinicaltrials” 2020n) |
Fig. 2.
Therapeutic agents ameliorating events propagating the COVID-19 infection
Anti-malarial drugs: chloroquine and hydroxychloroquine
An effective amine substitute of natural quinine, chloroquine, is an acid feeding quinine precursor, employed as the drug of choice against malaria (Parhizgar and Tahghighi 2017). Its hydroxyl analog, hydroxychloroquine, follows a similar pharmacokinetic profile, unlike their slightly different clinical considerations and toxicity agendas (Devaux et al. 2020). Chloroquine has more toxic effects than hydroxychloroquine, due to which it is either given in high doses/less duration or low doses/high duration (Devaux et al. 2020). Hydroxychloroquine has certain advantages like administration of high doses for longer duration and better tolerability. Chloroquine has a wide spectrum of activity against malaria, fungi, virus, and bacteria-mediated infections (Raoult et al. 1990). On account of its tolerability, cost effectiveness, limited toxicity profiles, and immunomodulatory properties, the effect of chloroquine was analyzed against multiple viruses (Bishop 1998; Boelaert et al. 2001; De et al. 2008; Dowall et al. 2015; Kronenberger et al. 1991; Mizui et al. 2010; Paton et al. 2011; Randolph et al. 1990; Tsiang and Superti 1984), where it is proved to be potent in blocking the replication cycle of the viruses. The synergistic effect of chloroquine with an antiviral drug, remdesivir, was reported to be potent against the COVID-19 in an investigation (Wang et al. 2020b), as declared by the China National Center for Biotechnology Development (Devaux et al. 2020). Chloroquine is being employed as the first-choice drug for infected patients in China and other parts of the globe, on account of its results in medical institutions and hospitals, immediately reducing fever, improving recovery time, and exhibiting minimum side effects as compared to the control in 100 infected subjects, where the clinical results depicted improved lung computed topography (LCT) images (Gao et al. 2020). The chloroquine-associated side effects like cardiomyopathy and macular retinopathy should be primarily considered via regular survey conduction, but this cannot overlook the impact chloroquine has on COVID-19 infections (Bernstein 1991). Chloroquine induces its anti-viral action via several mechanisms. The drug-mediated blockage of viral cycle is done either by interfering with the pathogen binding to their receptors, or by blocking replication, mediated by endosome, and dependent on pH (Tricou et al. 2010). Another action involves quinine reductase-2 inhibition, which is structurally similar to the sialic acid synthesizing enzyme, UDP-N-acetylglucosamine-2-epimerases (Kwiek et al. 2004; Varki 1997). In case of SARS-COV, chloroquine promoted glycolysation of ACE-2 on vero cells, which are the receptors for action of SARS-COV (Vincent et al. 2005). Moreover, modifying post-translational modification mechanisms of viral proteins is another action criteria of chloroquine against HIV, HSV, and dengue-2 virus (Randolph et al. 1990; Savarino et al. 1996). A number of clinical investigations have been conducted with both chloroquine and hydroxychloroquine (Zhang et al. 2020a). On account of these trials and possible mechanistic explanations, chloroquine phosphate is recommended to be administered orally at 500 mg dose (twice/day), 2 for adults, as registered in the Guidelines (version 6) (Dong et al. 2020). The therapeutic potential of hydroxychloroquine in COVID-19 infection has also been registered (NO: CHiCTR2000029559) and been investigated in 20 patients on 17th February. The 1–2 days of treatment recognized symptomatic relief followed by improved clinical lung features as in 19 patients after 5 days of hydroxychloroquine administration (Zhang et al. 2020b). The study concluded the short-term efficient role of hydroxychloroquine, leading to improved clinical features of the lungs, disease regression, and virus negative approach (Zhang et al. 2020b). The immune system action of chloroquine is another possibility of its employment in COVID-19 infection, through pro-inflammatory cytokine regulation and cell signaling. The chloroquine obstructs p38 mitogen-activated protein kinase (MAPK) phosphorylation in THP-1 cells as well as caspases-1, which is essential for viral replication cycle (Steiz et al. 2003). Moreover, its anti-inflammatory and immunomodulatory actions support its role in many other disorders. The drug also inhibits release of proinflammatory cytokines (Devaux et al. 2020). However, the drug has not yet completely permitted for public use (“Telegraphindia”).
Non-specific immunosuppressive and anti-inflammatory agents
The International guidelines for sepsis management allow the use of small doses of glucocorticoids for short time duration, in patients, unable to revive hemodynamic stability via appropriate fluid intake and vasopressor therapy (Rhodes et al. 2017). In order to conceal the serious symptoms (heart or kidney complications, ARDS) associated with COVID-19 infections, the glucocorticoid therapy is being employed currently. The patients with ARDS are recommended to use 1 to 2 mg of methylprednisolone/kg/day for a short duration (Chen et al. 2020a). Although, it was used as the primary drug in SARS epidemic, yet its use against COVID-19 has not yet been confirmed. Various studies depicted the protective role of glucocorticoids to mitigate the COVID-19 infection symptoms, early fever treatment and reduced pneumonia, but some indicated the drug to be ineffective, which needs to be evaluated further (Chen et al. 2006; Russell et al. 2020; Wang et al. 2020a; “WHO” 2020a).
Leflunomide is FDA-approved immunomodulatory prodrug, used for rheumatoid arthritis therapy, which is transformed to an active metabolite, A771726, following oral administration (Sarkar et al. 2020). The drug blocks tyrosine kinases and dihydroorotate dehydrogenase, as well as degradation of intracellular transcription factors. A single-center clinical investigation (phase 1) (NCT04361214) was conducted to evaluate the tolerability of leflunomide in COVID-19-infected (mild) ambulatory patients (Clinicaltrials.Gov 2020c). Another drug, thalidomide, blocks the excess generation of TNF-α and mitigate migration of leukocytes. Thalidomide was orally administered to 100 patients for 14 days or placebo, at the same dose in a multi-center, placebo-controlled, double-blind, phase 2 clinical trial (NCT04273529), to assess its efficacy and safety profile in COVID-19 Clinicaltrials.Gov 2020c). Furthermore, colchicine (alkaloid) is well-known for its use in inflammatory disorders. Colchicine or placebo (1:1) was administered to 6000 COVID-19-infected patients for 30 days, in controlled clinical investigation (phase 3; randomized, placebo-controlled, double-blind), to investigate the decrease in COVID-19-associated mortality rate and lung problems (“Clinicaltrials” 2020x). Ibuprofen (NSAID) blocks the generation of prostaglandins by ameliorating cyclooxygenase activity (Rogoveanu et al. 2018). A phase 4 clinical investigation was conducted (NCT04334629), to investigate the decrease in COVID-19-associated lung complications, by administration of 200 mg ibuprofen in 230 patients (Clinicaltrials.Gov 2020s). Moreover, naproxen is administered, either via oral or rectal route, for treatment of rheumatism and non-rheumatism conditions. It was investigated in a randomized clinical investigation, phase 3, (NCT04325633), with patients with severe COVID-19 were provided with standard care coupled with 250 mg BID ibuprofen and 30 mg/day lansoprazole, to determine its efficacy in COVID-19 (“Clinicaltrials” 2020d).
Anti-HCV, nucleotide inhibitors, repurposing agents
The role of anti-polymerase drugs is investigated on a COVID-19 RdRp model built via docking, modeling, and sequential analysis (Elfiky 2020). Human coronavirus is characterized by structural and non-structural proteins, where the former includes spike, matrix, envelope, and nucleocapsid and the latter involves RNA dependent RNA polymerase, which holds great significance in the coronavirus life cycle (Elfiky et al. 2017). Its active site comprises of a beta turn structure, bulging into two aspartate residues (Doublie and Ellenberger 1998). Favilavir (anti-polymerase drug) was approved recently after several conducted trials and studies (Elfiky 2020). Furthermore, directly acting anti-viral (DAA) drugs (sofosbuvir, ribavirin) are approved by FDA against RdRp of different types of viruses. They are engaged in competition for the active site of RdRp (Elfiky 2019). This category focuses on nucleotide inhibitors, which have a strong potential of inhibiting the viral RdRps (Biasini et al. 2014; Kirchdoerfer and Ward 2019; “NCBI”). Other compounds include sofosbuvir, IDX-184, ribavirin, Remdisivir, negative control compounds, cinnamaldehyde, thymoquinone, guanine triphosphate, and uracil triphosphate (Elfiky 2020). Before docking, structures of small molecules of the eight agents are prepared in optimized triphosphate form (Elfiky 2020). The interactions between RdRp active site and eight molecular ligands are studied and docking score values for SARS HCoV as well as COVID-19 are determined, in order to promote comparison between them. As a result, it was found that the binding energy for SARS HCoVs was greater than COVID-19, especially in IDX-184 and guanine triphosphate. To study the reason behind the difference in binding energies, the interaction complexes, generated post-docking, were analyzed. Therefore, the results depicted the formation of metallic interactions between IDX-184 and sofosbuvir, with D652 (two interactions) and E702 of COVID-19 RdRp, via magnesium ions. Moreover, sofosbuvir was found to form two interactions (hydrophobic) with D651 and Y510. These outcomes lead to the increased stabilization of IDX-184 and sofosbuvir, facilitating tight binding of these agents to the corona virus RdRp, thereby, eradicating the viral functioning. Between the two, IDX-184 is a more potent n-corona virus blocker and can be optimized further for more effective results (Elfiky 2020).
Antibodies and anti-parasitics
A phase 2, randomized, open-controlled, multi-center clinical investigation (NCT04286503) was carried out in 520 COVID-19 patients, with ritonavir/arbidol/chloroquine phosphate, for the active comparator group and carrimycin, for the experimental group, to evaluate the safety and efficacy of the drug (“Clinicaltrials” 2020v). Suramin sodium (reverse transcriptase enzyme-inhibitor) was administered in COVID-19-infected subjects, to evaluate its safety and efficacy profile (“Chictr” 2020d). Ivermectin is a broad-spectrum anti-parasitic and an anthelmintic drug, used to treat ectoparasitic diseases and onchocerciasis (“WHO” 2020a). The drug was administered in 60 subjects with severe COVID-19 disease, in a phase 2, randomized, clinical investigation (NCT04374279), with standard care, or bicalutamide-coupled standard care, for 1 week (150 mg/day) or 600 µg/kg of ivermectin for 3 days (Chen et al. 2020a, b). A fixed-dose antimalarial combination comprising of short-acting dihydroartemisinin (40 mg), and a long-acting piperaquine (320 mg), with lesser potency, is approved by the WHO for uncomplicated malaria (Amaratunga et al. 2016). The interaction between haem iron and peroxide bridge accounts for the antiviral potential of dihydroartemisinin/piperquine. Recently, the anti-COVID-19 potential of this drug is also assessed in 40 infected subjects (“Chictr” 2020a). Furthermore, azithromycin is used against a wide spectrum of gram-positive microorganisms (Călina et al. 2017). Orally administered azithromycin has been recently investigated as a prophylactic therapy, in a phase 2 clinical investigation (NCT04369365), in tumor patients, carrying out antineoplastic treatment during the pandemic (“Clinicaltrials” 2020q; Tran et al. 2019). A second-generation derivative of tetracycline, doxycycline (Blejan et al. 2020), which is investigated in a phase 3, randomized, multi-center, clinical study was conducted to compare the efficacy profile of 200 mg daily dose of doxycycline with placebo (lactose 380 mg/capsule) (“Clinicaltrials” 2020v).
Combination of arbidol and LPV/r versus LPV/r alone was a new treatment consideration based on the comparative study of patients administered with arbidol (anti-viral drug) in combination with lopinavir/ritonavir and the patients administered with lopinavir/ritonavir alone (Deng et al. 2020). The SARS-CoV-2 virus could not be identified in 75% of the group receiving the combination and 35% of the monotherapy group, after 7 days of administration. After 14 days, the virus was not detected in 94% of the combination group and 52.9% of monotherapy group. Moreover, the chest CT scan reports were improving in 69% of combination group and 29% of monotherapy candidates (Deng et al. 2020) (Table 2).
Table 2.
Result analysis of arbidol and LPV/r combinations and LPV/r alone
| Result | Combination group (oral administration of oral arbidol and LPV/r combination) | Monotherapy candidates (administered with oral LPV/r alone) |
|---|---|---|
| No SARS-CoV-2 detection after 7 days | 75% patients | 35% patients |
| No SARS-CoV-2 detection after 14 days | 94% patients | 52.9% patients |
| Improved chest CT scan reports | 69% patients | 295 patients |
Non-specific antiviral drugs
Immunoglobulin (Ig) blocks the activation of viral fragment crystallizable (Fc) receptor, which hinders infection progression, based upon antibody and stimulates the impact of endogenous neutralizing antibodies (Nabs) (Sarkar et al. 2020). Ig administered intravenously is used to elevate the treatment efficacy of COVID-19 infection, due to anti-inflammatory, immunomodulatory, and passive immunity induction ability (Sarkar et al. 2020). Owing to this, 80 subjects were administered with Ig via IV route in a phase 2/3 clinical investigation (NCT04261426), at 0.5 g/kg/day, for 5 days, to assess its efficacy and safety portfolio in the COVID-19 (“Chictr” 2020b). Interferon (INF)-β1a is used to treat multiple virus-mediated infections, by activation of enzymes in the cytoplasm, which hinders mRNA translation and production of proteins (Kamal et al. 2017). A research platform is recently provided by MJM Bonten for assessment of therapeutic efficacy of INF-β1a, against the COVID-19 disease in a phase 4 clinical investigation, NCT02735707, through intravenous administration, i.e., 10 µg/day for 6 days (“Clinicaltrials” 2020o). This cytokine was also investigated in 80 infected subjects, in a phase 2 clinical investigation (NCT04276688), with subcutaneous administration of 0.25 mg for 3 days to assess the decrease in mortality rate (Hung et al. 2020). The viral load was reported to be curbed by the combined therapy of INF-β-1a, with ribavirin and ritonavir/lopinavir, alongside the reduction in mortality rate, unlike the subjects administered only with lopinavir/ritonavir (“Clinicaltrials” 2020k). Interleukin-2 (IL-2) is an immunotherapeutic cytokine signaling molecule which prevents cancer and viral infections (Boda et al. 2018). This lymphocytotrophic hormone generates and regulates the immune response, which stimulates T cells for cell cycle progression, via multiple interactions with receptors, specific to the membrane (Sarkar et al. 2020). The generation of CD8 + T, CD4 + T, and NK cells is elevated in 20 infected patients at low intramuscular (IM) doses (“Clinicaltrials” 2020e).
Furthermore, the use of CYNK-001 has been approved by the US FDA, as a human placental CD34 + -derived experimental allogenic shelf cell therapy, to provide treatment to patients with COVID-19 (Celularity 2020). A global organization, “Cellularity Incorporated,” has recently investigated the safety and efficacy profile of CYNK-001 (NCT04365101) in 86 subjects, depicting that it has elevated for CD56 + /CD3 NK cells (“Clinicaltrials” 2020l). A first-in-class antiviral, baloxavir marboxil (S-033188), which is a prodrug, is converted to baloxavir acid (active form), through hydrolysis, which selectively inhibits cap-dependent endonuclease and neuraminidase inhibitors (NAIs) (Hayden et al. 2018; O’hanlon and Shaw 2019). This drug is specifically approved for treatment of influenza. This drug was recently reported to block cap-dependent endonuclease of the virus, after oral administration of 80 mg/day in 10 infected subjects (Lou et al. 2021).
Broad spectrum, RNA-dependent RNA polymerase inhibitors
An adenosine analog, remdesivir, is a monophosphoramidate prodrug (Lu 2020; Ren et al. 2020), which is metabolized into active form, GS-441524, inhibiting rial RNA production. Besides Ebola virus (Zhao et al. 2020), remdesivir has reported to inhibit corona viruses like MERS-CoV as well as SARS-CoV, significantly, as per in-vitro studies (“Coronavirus”; Holshue et al. 2020; Zhao 2020). A study involved 308 adult subjects, hospitalized with the COVID-19 infection, where the subjects were randomized to either remdesivir or placebo. A 200 mg LD of remdesivir was given on the first day, followed by further administration of IV doses (100 mg) daily for 9 days. The resulting outcome was depicted as the time taken for clinical recovery, up to 28 days (Lei et al. 2020).
Initially referred to as Avigan and T-705, favipiravir inhibits nonnucleoside RNA polymerase, selectively. An ongoing investigation on this drug is being carried out (NCT043336904) on 100 adult patients, infected with COVID-19, with 1800 mg/BID for first day, 600 mg/TID for second day, for 14 days at most, to promote its assessment and safety, alongside proper supportive care (“Clinicaltrials” 2020w). Arbidol (Blaising et al. 2014) is now being investigated for therapeutic safety and efficacy profile in the COVID-19 pandemic in phase 4 clinical investigations (NCT04246242), by administering adaptable oral doses of 200/400 mg/TID on 500 subjects (“Smartpatients” 2020). Furthermore, galidesivir is also being assessed on parameters of efficacy safety, tolerability, and pharmacokinetics, after IV administration, in comparison with placebo, in hospitalized patients with group A (yellow fever) or B (COVID-19) (“Clinicaltrials” 2020q).
Protease blocking anti-viral drugs (antiretrovirals)
An experimental nucleoside analog, azvudine, might inhibit the reverse enzyme transcriptase and depict efficacy against the COVID-19 disease (Wang et al. 2021), as being investigated in a phase 3 clinical investigation (ChiCTR2000029853) (“Chictr” 2020e). An inhibitor of HCV NS3/4A protease, danoprevir (Sarkar et al. 2020), is being investigated in a clinical investigation (phase 4), in combination with ritonavir, to assess its safety and efficacy profile in COVID-19 subjects, which has depicted prevention against viral transcription and replication (Chen et al. 2020a, b). The absorption of a non-peptidic protease inhibitor, darunavir, in combination with ritonavir (low dose), elevated, attaining peak plasma levels within 2.5–4 h (Sarkar et al. 2020). A phase 3, randomized clinical investigation (NCT04252274) is conducted to investigate the safety and efficacy profile of darunavir and cobicistat, recruiting SARS-CoV-2-infected patients (“Clinicaltrials” 2020b). Furthermore, TMC-310911 is similar to darunavir in structure and is a protease inhibitor, used to treat HIV-1 infections (Mina et al. 2020). An open-label trial (NCT04261907) is being conducted by Ascletis Pharmaceuticals Co., Ltd., of ASC09/ritonavir and lopinavir/ritonavir, which are investigated on 160 subjects, followed by their comparative analysis.
Vitamins and melatonin
Vitamin C is a popular antioxidant, which is used against inflammatory disorders, accumulation of neutrophils in lungs, respiratory failure, common cold, and as an immunomodulator. In addition to this, recently, multiple clinical investigations have been conducted to study the efficacy of vitamin C in COVID-19 (Sarkar et al. 2020). Vitamin C was administered intravenously to COVID-19 patients, in order to investigate its safety, efficacy, and tolerability in phase ½, non-randomized, open-label, clinical investigation (NCT04357782) (“Clinicaltrials” 2020t). Similarly, 18 years or aged COVID-19 patients with pneumonia were administered with vitamin C in another investigation (“Clinicaltrials” 2020s). Moreover, vitamin D acts against microbial peptide-induced viral replication, host cytokine storm, and renin-angiotensin system, via regulation of adaptive and innate immune system (Sarkar et al. 2020). Multiple investigations have portrayed a significant involvement of vitamin D in curbing COVID-19 symptoms. A randomized, phase 3 clinical trial (NCT04344041) has been conducted on 260 subjects with severe level of COVID-19 disease, administered with high or standard dose of vitamin D, coupled with 81 mg/day aspirin for 14 days (“Clinicaltrials” 2020h). The immunomodulatory impact of vitamin D renders it a potential role in the COVID-19 infection. The vitamin D synthesis is hindered by melanin (skin pigment) that encompasses a photoprotective ability (Islam et al. 2020). The people with dark skin tone possess higher concentration of melanin, which facilitate vitamin D synthesis inhibition in some people globally (Islam et al. 2020). Also, angiotensin II has been reported to propagate the process of melanogenesis. The SARS-CoV-2 enters the human lung epithelial cell, with the help of ACE-2 receptor. Therefore, a significant connection exists between angiotensin II, ACE-2, and melanogenesis. Elevated levels of angiotensin II decrease the synthesis of vitamin D (Islam et al. 2020). Taking into account the impact of multiple intrinsic and extrinsic factors, LIKE pigmentation (skin type), on the modulation of vitamin D, melanin polymers may also play a potential role in different disease outcomes within a diverse population (Sidiropoulou et al. 2020).
Melatonin is a hormone, which does not kill the virus, but instead blocks the replication cycle and entry events of the virus on account of its anti-inflammatory, anti-oxidant, and immune enhancing actions (Zhang et al. 2018). Curbed viral load, reduced fatality rate and viremia, and reduced paralysis are observed when melatonin is used against mice models with virus-infected CNS (Ben-Nathan et al. 1995). Reduced chances of reduced lung oxidative injury hindered release of pro-inflammatory cytokines and reduced inflammatory cell recruitment are effects of melatonin in respiratory syncytial virus models (Reiter et al. 2020). These outcomes suggested a possible involvement of melatonin in mitigating the progression of COVID-19 infection. It upregulates the immune system of the body, lowers the inflammatory responses and oxidative stress, and provides symptomatic relief to patients with respiratory complications (Zhang et al. 2018). As a potent anti-inflammatory agent, melatonin retards the production of pro-inflammatory cytokines, along with upregulation of IL-10 (an anti-inflammatory cytokine) (Habtemariam et al. 2017). Although, the anti-inflammatory potential of melatonin is quite clear, yet at high doses or lowered immune efficiency, melatonin poses a risk of increasing the level of pro-inflammatory cytokines, therefore, the possibility should be always considered (Carrascal et al. 2018). The anti-oxidant action of melatonin accounts for reduction in the levels of pro-oxidative enzymes, like NO synthase and enhanced production of anti-oxidative enzymes like superoxide dismutase (Reiter et al. 2020). Its role as free radical scavenger is also observed (Wu et al. 2019). Another possible target of melatonin action is Toll-like receptors 4 (TLR4), which causes rapid production of IL-6 alveolar macrophages, as a result of immune activation by LDL in SARS-induced models (Imai et al. 2008). In severe conditions, inflammation, hypoxemia, and ventilation with high oxygen levels are enhanced, favoring excessive oxidation in the infected patients (Tamura et al. 2002). Therefore, the anti-oxidant potential of melatonin is quite applicable in such cases. The virus, upon entry, deteriorates the respiratory epithelial cells, following which the antigens are phagocytosed by dendritic cells and presented to effector T cells which kill the infected cells and CD8 + T cells mediated cell apoptosis (Rogers and Williams 2018). The pathogenic entry and cell apoptosis activates the immune system against the infection. Melatonin promotes upregulation of the immune system by maturation of B and T lymphocytes, monocytes, and granulocytes (Zhang et al. 2018). Furthermore, the anti-viral impact of numerous dietary supplements, like garlic, orange, black seed, ginger, omega-3 polyunsaturated fatty acids (PUFAs), cranberry, omega-6 PUFAs, minerals (Mg, Mn, Cu, Na, Fe, Zn, Se), and vitamins (A, B, C, D, E), renders them effects against multiple species of respiratory viruses, including the SARS-viruses (Islam et al. 2021b). Thus, dietary supplements, such as probiotics, vitamins and minerals, and individual nutrition pattern, can be employed as an adjunct therapy in combination with the antiviral medication, to hinder the progression of the COVID-19 disease (Islam et al. 2021b).
JAK inhibitors
ACE2 is a protein entity existing on cells in kidney, blood vessels, lungs, and heart that might be considered a receptor for novel corona virus. AP2-associated protein kinase 1 (AAK1)-mediated endocytosis is the possible mechanism of entry of COVID-19 in the human body (Zhang et al. 2020b). Therefore, agents capable of inhibiting AAK1 can hinder the entry mechanisms of the virus by inhibiting endocytosis process. Thus, one of the agents under this category involve baricitinib, which is also an AAK1 and a JAK inhibitor, exhibiting high affinity and safety profiles at therapeutic doses (2–4 mg per day), which promote its positive role in treatment of COVID-19 infections (Richardson et al. 2020). Despite the protective influence of JAK inhibitors in COVID-19 infections, the agents are associated with a primary drawback based on their ability to constrain inflammatory cytokine release along with INF-a, which on the contrary has an anti-viral approach (Zhang et al. 2020b). There are a couple of registered clinical investigations that have been carried out to evidently support the potential of these agents in curbing the infection events. “Study for safety and efficacy of Jakotinib hydrochloride tablets in the treatment of severe and acute exacerbation patients of novel coronavirus pneumonia” (CHiCTR2000030170) is a registered Chinese clinical trial targeting the optimized effect of jakotinib, an oral JAK1, JAK2, and JAK3 inhibitor used for cancer, myofibrosis, and autoimmune problems (Zhang et al. 2020b). Fifty in-patients with acute and severe levels of COVID-19 infection were selected for the trials to support the positive results of the drug in mitigating the infection events. Another study involved investigation of a positive correlation between ruxolitinib along with mesenchymal stem cells and mitigation of coronavirus infection in patients with severely infected patients (ChiCTR2000029580) (Zhang et al. 2020b).
Convalescent plasma therapy
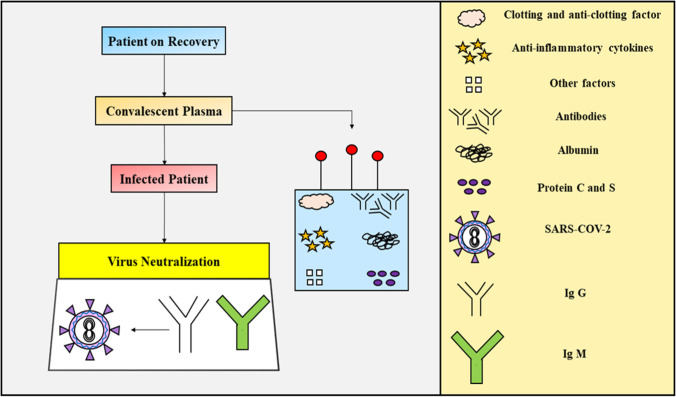
Recently, the Sree Chitra Tirunal Institute for Medical Sciences and Technology (SCTIMST) has come up with an innovative treatment approach to treat COVID-19 patients, significantly known as convalescent plasma therapy (Fig. 3), aiming at the treatment utilizing immune strength gained by the recovered patient, to treat a diseased individual. The Indian Council for Medical Research (ICMR), being the topmost authorizing body in the country, has given approval to SCTIMST for carrying out the novel treatment. The immune system of the body is designed to produce antibodies, when corona virus like pathogens attacks it (“Inbministry”). These antibodies identify and mark the viral particles invading our biological system. WBCs soon activate the defense mechanism of the body, which gets rid of the infection. Blood transfusion like techniques harvests the antibodies from a recovered individual, administering it into a sick patient, to fight the invading virus (“Inbministry”). A healthy subject who has recovered from the COVID-19 infection has been selected and the blood is drawn from the subject, which too after proper examination, where the swab test should be negative, with the donor declared cures, followed by either 2 weeks waiting or making sure the subject be asymptomatic for at least 28 days. From the blood, the serum is separated and screened for antibodies neutralizing the viral particles and the blood serum which is obtained from the recovered patient is rich in viral neutralizing antibodies which is called convalescent serum and is administered to the diseased/infected patients, due to which the patient acquires passive immunization (“Inbministry”). The clinical analysis portfolio requires administration in a small group of patients first and is restricted to be used for only severely ill patients, presently and this method is based upon passive immunization in the vaccinated person, producing antibodies in the immune system whenever the infection hits the body. Whenever a new viral outbreak occurs, the drugs are always limited as there are limited antibiotics available, unlike bacterial infections. This plasma therapy has been used in the past, like during the H1N1 influenza virus pandemic (2009–2010), where the serum-treated individuals showed significant improvement and the same process exhibited potent results in the Ebola outbreak in 2018 (“Inbministry”).
Fig. 3.
Schematic representation of the convalescent plasma therapy in COVID-19
This technology is associated with certain challenges also, which must be taken into consideration, as it holds great medical significance in the present times. The primary concern is the safety profile of this treatment. In the current world of technological advancement, the screening of blood-borne pathogens and matching blood type of donors and recipients is not a difficult approach, and also the risks for transferring the infectious agents or triggering transfusion reactions are low. However, a primary issue with the plasma therapies is that these treatment therapies are temporary arrangements, unlike normal vaccinations, and do not provide lifelong immunity, as the passive immunization only lasts up to the time the antibodies injected remain into the bloodstream (i.e., 3–4 days). The USA and Chinese reports reveal that the benefits of plasma transfusion are obtained only in the first 3–4 days and not later (Richardson et al. 2020). Furthermore, other challenges include difficulty to obtain sufficient amount of plasma for the recovered individuals, and also not all patients can volunteer to donate blood, on account of deteriorated medical conditions. Microsoft is currently working with US-based pharmaceutical firms and will soon launch a chatbot to encourage plasma donation by recovered COVID-19 patients and this “plasmabot” will aid in determining, whether the donor is eligible for donation, further directing them to nearby sites.
Future prospects and conclusion
The COVID-19 pandemic is associated with high proinflammatory disease states, via induction of lower levels of cytokine expression, with a moderate reaction of INF-stimulated genes (ISGs) and elevating the expression of chemokines. Various new treatment strategies for viral infections are verified and studied using animal models, which also reveal the pathogenic outcomes of viral progression in human body. Therefore, selection of a suitable model for testing the treatment efficacy is the primary concern of the researchers worldwide. Even though the zoonotic source of SARS-CoV-2 is not yet confirmed, yet its genomic sequential analysis depicts its similarity with the SARS-CoV. All these phylogenetic evidences help the researchers to formulate effective outcomes, which would help them to study the SARS-CoV-2 features. Various treatment strategies have already been formulated based on potent clinical evidences. Triage screening is an effective screening technique characterized with screening of patients at medical facilities, based upon the symptoms and the history of their exposures, travel history, or residential proximity to infection that might provide an evident link between the patient and COVID-19 infection. In developing countries like India, a major problem faced by the medical institutions is lack of ventilators for the patients. This is a serious issue that needs to be resolved, before the viral infection reaches the third stage. The poor people visualize threat to livelihood as a major concern instead of threat to life. Therefore, there should have proper provisions for these people, to facilitate continuous supply of food and basic necessities in their approach. Many countries have taken adequate steps to mitigate such problems, like nationalization of private hospitals in Spain, and USA promising thousands of dollars for such under privileged citizens. Notably, community and home care provide reliable strategies to fight the horrors of the pandemic. For instance, Greece focused on hospital preparedness, however, could not reinforce primary and community healthcare. In combination with errors in epidemiological surveillance, a second, strict, horizontal lockdown was implemented in Greece, and Europe experiences one of the highest death rates during the second wave of the pandemic (Farsalinos et al. 2021). Therefore, primary and community care should be targeted to manage the public health crisis as well as prevent unnecessary admissions to the hospitals. This, along with more selective social distancing measures, we can provide a reliable as well as long-term solution, facilitation COVID-19 amelioration (Farsalinos et al. 2021).
Also, the development and progression of genetic variants of SARS-CoV-2, like B.1.1.7, B.1.351, B.1.617.2, P.1, B.1.525, B.1.526, B.1.617.1, B.1.1.529, and so on, has led to greater transmission as well as propagation of the virus in the population. In order to manage this, firstly, the genomic surveillance program should be comprehensive, for instance Genomic UK, and should have strong ties among entities performing SARS-CoV-2 diagnosis; laboratories with resources, capacity, and expertise for the conduction of large-scale sequencing; and computation experts to analyze large genomic datasets (Grubaugh et al. 2021). However, lack of infrastructure, political support, and technical expertise limits the availability of genomic surveillance in multiple countries (Grubaugh et al. 2021). Thus, being a global concern, the virus should be collectively targeted by nations worldwide, and this community should provide financial and technical support to strengthen genomic surveillance in less-privileged areas. Also, standard PCR assays can also promote detection of virus, during unavailability of large-scale sequencing (Grubaugh et al. 2021). Table 3 gives an overview of the bottlenecks identified in the COVID-19 pandemic, which might provide future assistance in better and effective management of a pandemic.
Table 3.
A brief description of lessons learnt from the COVID-19 pandemic of 2019
| Current issues | Events | Consequences | Lessons learnt |
|---|---|---|---|
| Delay in travel restrictions | Aviation services prevailed till a month after the outbreak with negligible screening focus at the airports | People from China entered multiple countries and transmitted the infection | Screening of citizens at the airport is extremely important and should be implemented at the earliest |
| Misinformation among the public | Rumors and misinformation due to lack of transparency | Racism and unsuitable precautions prevailed | Transparency as well as open access to all information is necessary |
| Lack of transparency | Intimidation of health professionals who recognized the disease initially | Information release delay | Clear, transparent policies should be developed |
| Emergency announcement delay | The declaration of the health emergency, globally, was done on 30th January, 2020, a month late from the China outbreak | The disease severity was not able to reach the globe on time | The treatment potential should be broadcasted earlier |
| Quarantine delay | Quarantine was delayed by one whole month in China | Rapid infection spread | Quarantine the susceptible areas as soon as possible |
| R & D | Lack of funding | A global pandemic has occurred causing deaths of thousands of people | Proper investigation and R & D protocol needs to be followed |
| Problems with healthcare management | Even the best hospital system could not facilitate viral control | Rapid transmission | Primary and community health care should be implemented |
| Viral mutations | Development of genetic variants | Elevation transmission and propagation | Comprehensive genomic surveillance program should be implemented; resourceful labs with expert staff; Computation tools and techniques |
The problems associated with poverty and hunger needs, limited number of hospitals and equipment, inadequacy in availability of medical professionals, lack of awareness, and economic obstacles can be dampened in the future. Taking effective preventive measures and adopting a healthy lifestyle is a prior treatment plan that needs to be followed. Resolving all the problems and challenges discussed above, providing adequate funds for researches and trials, strengthening the national economic sector, and adopting a healthy lifestyle can help us combat such viral pathogens and grant us emotional, physical, economical, and mental health, in order to save lives across the globe and promoting international harmony. The manuscript targets to disseminate a comprehensive data about the possible therapeutic targets and drug candidates, which might provide relief to the infected individuals and simultaneously providing the researchers a head start towards finding a reliable and permanent solution for the COVID-19.
Acknowledgements
The authors would like to thank Chitkara College of Pharmacy, Chitkara University, Punjab, India for providing facilities for completion of this review.
Author contribution
IK and TB conceived the study and wrote the first draft of the paper; AS, SS, and NS: figure work; VS, SF, NKF, and MS: data compilation; HGD, AMA, and SB: editing; AAH, LA, and SBU: proof read.
Data availability
Not applicable.
Declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
All the authors have approved the manuscript for publication.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Adnan SM, Khan S, Kazmi A, Bashir N, Siddique R. COVID-19 infection: Emergence, transmission, and characteristics of human coronaviruses. J Adv Res. 2020;24:91–98. doi: 10.1016/j.jare.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alhazzani W, Møller MH, Arabi YM, Loeb M, Gong MN, Fan E, Oczkowski S, Levy MM, Derde L. A Dzierba, B Du, Surviving sepsis campaign: guidelines on the management of critically Ill adults with coronavirus disease 2019 (COVID-19) Intensive Care Med. 2020;46:854–887. doi: 10.1007/s00134-020-06022-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaratunga C, Lim P, Suon S, Sreng S, Mao S, Sopha C, Sam B, Dek D, Try V, Amato R, Blessborn D. Dihydroartemisinin-piperaquine resistance in Plasmodium falciparum malaria in Cambodia: a multisite prospective cohort study. Lancet Infect Dis. 2016;16:357–365. doi: 10.1016/S1473-3099(15)00487-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assiri A, Al-Tawfiq JA, Al-Rabeeah AA, Al-Rabiah FA, Al-Hajjar S, Al-Barrak A, Flemban H, Al-Nassir WN, Balkhy HH, Al-Hakeem RF, Makhdoom HQ. Epidemiological, demographic, and clinical characteristics of 47 cases of Middle East respiratory syndrome coronavirus disease from Saudi Arabia: a descriptive study. Lancet Infect Dis. 2013;13:752–761. doi: 10.1016/S1473-3099(13)70204-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Nathan B, Maestroni GJ, Lustig S, Conti A. Protective effects of melatonin in mice infected with encephalitis viruses. Arch Virol. 1995;140:223–230. doi: 10.1007/BF01309858. [DOI] [PubMed] [Google Scholar]
- Bernstein HN. Ocular safety of hydroxychloroquine. Ann Ophthalmol. 1991;23:292–296. [PubMed] [Google Scholar]
- Biasini M, Bienert S, Waterhouse A, Arnold K, Studer G, Schmidt T, Kiefer F, Cassarino TG, Bertoni M, Bordoli L, Schwede T. SWISS-MODEL: modelling protein tertiary and quaternary structure using evolutionary information. Nucleic Acids Res. 2014;42:W252–W258. doi: 10.1093/nar/gku340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop NE. Practical guidelines in antiviral therapy. Intervirology. 1998;41:261–271. doi: 10.1159/000024948. [DOI] [PubMed] [Google Scholar]
- Blaising J, Polyak SJ, Pécheur EI, Arbidol as a broad-spectrum antiviral: an update. Antiviral Res. 2014;107:84–94. doi: 10.1016/j.antiviral.2014.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blejan IE, Diaconu CC, Letiția AN, Arsene DI, Ghica MA, Drăgănescu DO, Dragomiroiu GT, Rădulescu M, Maltezou HC, Tsatsakis AM, Papasavva M. Antibiotic resistance in community-acquired pneumonia. A Romanian Perspective Farmacia. 2020;68:512–520. [Google Scholar]
- Boda D, Docea AO, Calina D, Ilie MA, Caruntu C, Zurac S, Neagu M, Constantin C, Branisteanu DE, Voiculescu V, Mamoulakis C. Human papilloma virus: apprehending the link with carcinogenesis and unveiling new research avenues (Review) Int J Oncol. 2018;52:637–655. doi: 10.3892/ijo.2018.4256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boelaert JR, Piette J, Sperber K. The potential place of chloroquine in the treatment of HIV-1-infected patients. J Clin Virol. 2001;20:137–140. doi: 10.1016/S1386-6532(00)00140-2. [DOI] [PubMed] [Google Scholar]
- Călina D, Docea AO, Rosu L, Zlatian O, Rosu AF, Anghelina F, Rogoveanu O, Arsene AL, Nicolae AC, Drăgoi CM, Tsiaoussis J (2017) Antimicrobial resistance development following surgical site infections. Mol Med Rep 15:681–688 [DOI] [PMC free article] [PubMed]
- Cao B, Wang Y, Wen D, Liu W, Wang J, Fan G, Ruan L, Song B, Cai Y, Wei M, Li X A (2020a) Trial of lopinavir–ritonavir in adults hospitalized with severe Covid-19. New Engl J Med 382:1787–1799 [DOI] [PMC free article] [PubMed]
- Cao W, Liu X, Bai T, Fan H, Hong K, Song H (2020b) High-dose intravenous immunoglobulin as a therapeutic option for deteriorating patients with coronavirus disease 2019. Open Forum Infect Dis 7:102 [DOI] [PMC free article] [PubMed]
- Carlos WG, Dela CS, Cao B, Pasnick S, Jamil S. Novel wuhan (2019-nCoV) coronavirus. Am J Respir Crit Care Med. 2020;201:7–8. doi: 10.1164/rccm.2014P7. [DOI] [PubMed] [Google Scholar]
- Carrascal L, Nunez-Abades P, Ayala A, Cano M. Role of melatonin in the inflammatory process and its therapeutic potential. Curr Pharm Design. 2018;24:1563–1588. doi: 10.2174/1381612824666180426112832. [DOI] [PubMed] [Google Scholar]
- CDC (2020a) Novel Coronavirus (2019a-nCoV) Pregnant women. Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019a-ncov/specificgroups/pregnancy-faq.html. Accessed 19 Mar 2020
- CDC (2020b) Novel Coronavirus (2019b-nCoV) Situation summary. Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019b-ncov/summary.html. Accessed 18 Mar 2020
- CDC: Coronavirus Disease (2020c) (COVID-19): Interim Considerations for Infection Prevention and Control of Coronavirus Disease 2019a (COVID-19) in Inpatient Obstetric Healthcare Settings. CDC website. Updated February 27, 2020. Reviewed February 18, 2020. https://www.cdc.gov/coronavirus/2019a-ncov/hcp/inpatient-obstetric-healthcare-guidance.html#anchor_1582067966715. Accessed 23 Mar 2020
- CDC: Coronavirus Disease (2020d) (COVID-19): Interim Guidance for Implementing Home Care of People Not Requiring Hospitalization for 2019b Novel Coronavirus (2019b-nCoV). CDC website. Updated March 20, 2020. Reviewed March 20, 2020. https://www.cdc.gov/coronavirus/2019-ncov/hcp/guidance-home-care.html. Accessed 23 Mar 2020
- Celularity (2020) Celularity announces FDA clearance of IND application for CYNK001 in coronavirus, first in cellular therapy. Available at: https://clinicaltrials.gov/ct2/show/NCT04365101. Accessed 30 June 2020
- Chavez S, Long B. A Koyfman, SY Liang, Coronavirus disease (COVID-19): a primer for emergency physicians. Am J Emerg Med. 2020;44:220–229. doi: 10.1016/j.ajem.2020.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Zhang Z, Wang L, Huang Z, Gong F, Li X, Chen Y, Wu JJ (2020a) First clinical study using HCV protease inhibitor danoprevir to treat naive and experienced COVID-19 patients. MedRxiv 99:e2357 [DOI] [PMC free article] [PubMed]
- Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, Qiu Y, Wang J, Liu Y, Wei Y, Yu T. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen RC, Tang XP, Tan SY, Liang BL, Wan ZY, Fang JQ, Zhong N. Treatment of severe acute respiratory syndrome with glucosteroids: the Guangzhou experience. Chest. 2006;129:1441–1452. doi: 10.1378/chest.129.6.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chictr.Org.Cn (2020a) Cancelled by the investigator randomized controlled trial for the efficacy of dihydroartemisinine piperaquine in the treatment of mild/ common novel coronavirus pneumonia (COVID-19). Available at: http://www.chictr.org.cn/showproj.aspx?proj=49915.Accessed Mar 15 2020a
- Chictr.Org.Cn (2020b) Clinical trial for recombinant human interleukin-2 in the treatment of novel coronavirus pneumonia (COVID-19). Available at: http:// www.chictr.org.cn/showprojen.aspx?proj=49567. Accessed Feb 24 2020b
- Chictr.Org.Cn (2020d) A multi-center study on the efficacy and safety of suramin sodium in adult patients with novel coronavirus pneumonia (COVID-19). Available at: http://www.chictr.org.cn/showprojen.aspx?proj=49824. Accessed Feb 21 2020d
- Chictr.Org.Cn (2020e) A randomized, open-label, controlled clinical trial for azvudine in the treatment of novel coronavirus pneumonia (COVID-19). Available at: http://www.chictr.org.cn/historyversionpuben.aspx?regno=ChiCTR2000030424. Accessed May 14 2020e
- Clinicaltrials.Gov (2020a) Efficacy and safety of corticosteroids in COVID-19. Available at: https://clinicaltrials.gov/ct2/show/NCT04273321. Accessed May 11 2020a
- Clinicaltrials.Gov (2020b). Efficacy and safety of darunavir and cobicistat for treatment of COVID-19 (DC-COVID-19), Available at: https://clinicaltrials.Gov/ct2/show/NCT04252274. Accessed Apr 13 2020b
- Clinicaltrials.Gov (2020c) The efficacy and safety of thalidomide in the adjuvant treatment of moderate new coronavirus (COVID-19) pneumonia. Available at: https://clinicaltrials.gov/ct2/show/NCT04273529. Accessed Feb 21 2020c
- Clinicaltrials.Gov (2020d) Efficacy of addition of naproxen in the treatment of critically Ill patients hospitalized for COVID-19 infection (ENACOVID). Available at: https://clinicaltrials.gov/ct2/show/NCT04325633. Accessed Apr 14 2020d
- Clinicaltrials.Gov (2020e) The efficacy of intravenous immunoglobulin therapy for severe 2019-nCoV infected pneumonia. Available at: https://clinicaltrials.gov/ct2/show/NCT02543567. Accessed Feb 7 2020e
- Clinicaltrials.Gov (2020f) Evaluating and comparing the safety and efficiency of ASC09/ritonavir and lopinavir/ritonavir for novel coronavirus infection. Available at: https://clinicaltrials.gov/ct2/show/NCT04261907 (Accessed 2020f Feb 10)
- Clinicaltrials.Gov (2020g) International ALLIANCE study of therapies to prevent progression of COVID-19. Available at: https://clinicaltrials.gov/ct2/show/NCT04395768. Accessed May 20 2020g
- Clinicaltrials.Gov (2020h) The LEAD COVID-19 trial: low-risk, early aspirin and vitamin D to reduce COVID-19 hospitalizations (LEAD COVID-19). Available at: https://clinicaltrials.gov/ct2/show/NCT04363840. Accessed May 6 2020h
- Clinicaltrials.Gov (2020i) Leflunomide in mild COVID-19 patients. Available at: https://clinicaltrials.gov/ct2/show/NCT04361214. Accessed May 11 2020i
- Clinicaltrials.Gov (2020j) LIBERATE Trial in COVID-19 (LIBERATE). Available at: https://clinicaltrials.gov/ct2/show/NCT04334629. Accessed May 1 2020j
- Clinicaltrials.Gov (2020k) Lopinavir/ ritonavir, ribavirin and IFN-beta combination for nCoV treatment. Available at: https://clinicaltrials.gov/ct2/show/NCT04276688. Accessed Apr 15 2020k
- Clinicaltrials.Gov (2020l) Natural Killer Cell (CYNK-001) Infusions in adults with COVID-19 (CYNK-001-COVID-19). Available at: https://clinicaltrials.gov/ct2/show/NCT04365101. Accessed Apr 28 2020l
- Clinicaltrials.Gov (2020m) Post-exposure prophylaxis / preemptive therapy for SARScoronavirus-2 (COVID-19 PEP). Available at: https://clinicaltrials.gov/ct2/show/NCT04308668. Accessed May 1 2020m
- Clinicaltrials.Gov (2020n) Randomized proof-of-concept trial to evaluate the safety and explore the effectiveness of resveratrol for COVID-19. Available at: https://clinicaltrials.gov/ct2/show/NCT04400890. Accessed May 26 2020n
- Clinicaltrials.Gov (2020o). Randomized, embedded, multifactorial adaptive platform trial for community- acquired pneumonia (REMAP-CAP). Available at: https://clinicaltrials.gov/ct2/show/NCT02735707. Accessed Apr 16 2020o [DOI] [PMC free article] [PubMed]
- Clinicaltrials.Gov (2020q) A study to evaluate the safety, pharmacokinetics and antiviral effects of galidesivir in yellow fever or COVID-19. Available at: https://clinicaltrials.gov/ct2/show/NCT03891420. Accessed Apr 15 2020q
- Clinicaltrials.Gov (2020r) Trial to promote recovery from COVID-19 with ivermectin or endocrine therapy (RECOVER). Available at: https://clinicaltrials.gov/ct2/show/NCT04374279. Accessed May 5 2020p
- Clinicaltrials.Gov (2020s) Vitamin C infusion for the treatment of severe 2019- nCoV infected pneumonia. Available at: https://clinicaltrials.gov/ct2/show/NCT04264533 (Accessed 2020s Mar 10)
- Clinicaltrials.Gov (2020t) Administration of intravenous vitamin C in novel coronavirus infection (COVID-19) and decreased oxygenation (AVoCaDO). Available at: https://clinicaltrials.gov/ct2/show/NCT04357782. Accessed Apr 22 2020q
- Clinicaltrials.Gov (2020u) Cholecalciferol to improve the outcomes of COVID-19 patients (CARED). Available at: https://clinicaltrials.gov/ct2/show/NCT04411446. Accessed Jun 4 2020r
- Clinicaltrials.Gov (2020v) Available at: https://clinicaltrials.gov/ct2/show/NCT04286503 (Accessed the Clinical Study of Carrimycin on Treatment Patients with COVID-192020v Feb 27)
- Clinicaltrials.Gov (2020w) Clinical study to evaluate the performance and safety of favipiravir in COVID-19. Available at: https://clinicaltrials.gov/ct2/show/NCT04336904. Accessed Apr 8 2020s
- Clinicaltrials.Gov (2020x) Colchicine coronavirus SARS-CoV2 trial (COLCORONA) (COVID-19). Available at: https://clinicaltrials.gov/ct2/show/NCT04322682. Accessed 24 Apr 2020
- Clinicaltrials.Gov (2020y) COvid-19 and vitamin D supplementation: a multicenter randomized controlled trial of high dose versus standard dose vitamin D3 in highrisk COVID-19 patients (CoVitTrial). Available at: https://clinicaltrials.gov/ct2/show/NCT04344041. Accessed 6 May 2020
- Cole GM, Frautschy SA. Mechanisms of action of non-steroidal anti-inflammatory drugs for the prevention of Alzheimer’s disease. CNS Neurol Disord Drug Targets. 2010;9:140–148. doi: 10.2174/187152710791011991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui J, Li F, Shi ZL. Origin and evolution of pathogenic coronaviruses. Nat Rev Microbiol. 2019;17:181–192. doi: 10.1038/s41579-018-0118-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De XL, Boisson V, Reynier JC, Enault S, Charrel RN, Flahault A, et al. On Chikungunya acute infection and chloroquine treatment. Vector Borne Zoonotic Dis. 2008;8:837–840. doi: 10.1089/vbz.2008.0049. [DOI] [PubMed] [Google Scholar]
- Deng L, Lia C, Zeng Q, Liu X, Li X, Zhang H, Hong Z, Xia J. Arbidol combined with LPV/r versus LPV/r alone against corona virus disease 2019: a retrospective cohort study. J Infect. 2020;81:e1–5. doi: 10.1016/j.jinf.2020.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devaux CA, Rolain JM, Colson P, Raoult D (2020) New insights on the antiviral effects of chloroquine against coronavirus: what to expect for COVID-19? Int J Antimicrob Agents 55:105938 [DOI] [PMC free article] [PubMed]
- Dong L, Hu S, Gao J. Discovering drugs to treat coronavirus disease 2019 (COVID-19) Drug Discov Ther. 2020;14:58–60. doi: 10.5582/ddt.2020.01012. [DOI] [PubMed] [Google Scholar]
- Dorward J, Gbinigie K (2020) Lopinavir/ritonavir: a rapid review of effectiveness in COVID-19. 19
- Doublie S, Ellenberger T. The mechanism of action of T7 DNA polymerase. Curr Opin Struct Biol. 1998;8:704–712. doi: 10.1016/S0959-440X(98)80089-4. [DOI] [PubMed] [Google Scholar]
- Dowall SD, Bosworth A, Watson R, Bewley K, Taylor I, Rayner E, Hunter L, Pearson G, Easterbrook L, Pitman J, Hewson R. Chloroquine inhibited Ebola virus replication in vitro but failed to protect against infection and disease in the in vivo guinea pig model. J Gen Virol. 2015;96:3484–3492. doi: 10.1099/jgv.0.000309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elfiky AA. A Ismail, Molecular dynamics and docking reveal the potency of novel GTP derivatives against RNA dependent RNA polymerase of genotype 4a HCV. Life Sci. 2019;238:116958. doi: 10.1016/j.lfs.2019.116958. [DOI] [PubMed] [Google Scholar]
- Elfiky AA. Anti-HCV, nucleotide inhibitors, repurposing against COVID-19. Life Sci. 2020;248:117477. doi: 10.1016/j.lfs.2020.117477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elfiky AA, Mahdy SM, Elsheme WM. Quantitative structure-activity relationship and molecular docking revealed a potency of anti-hepatitis C virus drugs against human corona viruses. J Med Virol. 2017;89:1040–1047. doi: 10.1002/jmv.24736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farsalinos K, Poulas K, Kouretas D, Vantarakis A, Leotsinidis M, Kouvelas D, Docea AO, Kostoff R, Gerotziafas GT, Antoniou MN, Polosa R, Barbouni A, Yiakoumaki V, Giannouchos TV, Bagos PG, Lazopoulos G, Izotov BN, Tutelyan VA, Aschner M, Hartung T, Wallace HM, Carvalho F, Domingo JL, Tsatsakis A. Improved strategies to counter the COVID-19 pandemic: lockdowns vs. primary and community healthcare. Toxicol Rep. 2021;8:1–9. doi: 10.1016/j.toxrep.2020.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuta Y, Komeno T, Nakamura T. Favipiravir (T-705), a broad spectrum inhibitor of viral RNA polymerase. Proc Japan Acad Ser B. 2017;93:449–463. doi: 10.2183/pjab.93.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao J, Tian Z, Yang X. Breakthrough: chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. Biosci Trends. 2020;14:72–73. doi: 10.5582/bst.2020.01047. [DOI] [PubMed] [Google Scholar]
- Grubaugh ND, Hodcroft EB, Fauver JR, Phelan AL, Cevik M. Public health actions to control new SARS-CoV-2 variants. Cell. 2021;184(5):1127–1132. doi: 10.1016/j.cell.2021.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habtemariam S, Daglia M, Sureda A, Selamoglu Z, Gulhan MF, Nabavi SM. Melatonin and respiratory diseases: a review. Curr Top Med Chem. 2017;17:467–488. doi: 10.2174/1568026616666160824120338. [DOI] [PubMed] [Google Scholar]
- Harrison C. Coronavirus puts drug repurposing on the fast track. Nat Biotechnol. 2020;38:379–381. doi: 10.1038/d41587-020-00003-1. [DOI] [PubMed] [Google Scholar]
- Hayden FG, Sugaya N, Hirotsu N, Lee N, de Jong MD, Hurt AC, Ishida T, Sekino H, Yamada K, Portsmouth S, Kawaguchi K. Baloxavir marboxil for uncomplicated influenza in adults and adolescents. N Engl J Med. 2018;379:913–923. doi: 10.1056/NEJMoa1716197. [DOI] [PubMed] [Google Scholar]
- Holshue ML, DeBolt C, Lindquist S, Lofy KH, Wiesman J, Bruce H, Spitters C, Ericson K, Wilkerson S, Tural A, Diaz G. First case of 2019 novel coronavirus in the United States. N Engl J Med. 2020;382:929–986. doi: 10.1056/NEJMoa2001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z. Clinical features of patients infected with the 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung IFN, Lung KC, Tso EYK, Liu R, Chung TWH, Chu MY, Ng YY, Lo J, Chan J, Tam AR, Shum HP. Triple combination of interferon beta-1b, lopinavir–ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID-19: an open-label, randomised, phase 2 trial. Lancet. 2020;395:1695–1704. doi: 10.1016/S0140-6736(20)31042-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huynh J, Li S, Yount B, Smith A, Sturges L, Olsen JC, Nagel J, Johnson JB, Agnihothram S, Gates JE, Frieman MB. Evidence sup porting a zoonotic origin of human coronavirus strain NL63. J Virol. 2012;86:12816–12825. doi: 10.1128/JVI.00906-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai Y, Kuba K, Neely GG, Yaghubian-Malhami R, Perkmann T, van Loo G, Ermolaeva M, Veldhuizen R, Leung YC, Wang H, Liu H. Identification of oxidative stress and Toll-like receptor 4 signaling as a key pathway of acute lung injury. Cell. 2008;133:235–249. doi: 10.1016/j.cell.2008.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam MT, Salehi B, Karampelas O, Sharifi-Rad J, Docea AO, Martorell M, Calina D. High skin melanin content, vitamin D deficiency and immunity: potential interference for severity of COVID-19. Farmacia. 2020;68:6. doi: 10.31925/farmacia.2020.6.3. [DOI] [Google Scholar]
- Islam MT, Quispe C, Herrera-Bravo J, Khan I, et al. Possible mutation pathways in SARS-CoV-2. Farmacia. 2021;69:6. doi: 10.31925/farmacia.2021.6.1. [DOI] [Google Scholar]
- Islam MT, Quispe C, Martorell M, Docea AO, Salehi B, Calina D, Reiner Ž, Sharifi-Rad J (2021b) Dietary supplements, vitamins and minerals as potential interventions against viruses: perspectives for COVID-19. International J Vitam Nutr Res 92. 10.1024/0300-9831/a000694 [DOI] [PubMed]
- Kamal AM, Mitru P, Docea AO, Sosoi SS, Kamal CK, Mitrut R, Margaritescu D, Calina D, Banciu C, Tica OS, Tica AA. Double therapy with pegylated interferon and ribavirin for chronic hepatitis C. A pharmacogenetic guide for predicting adverse events. Farmacia. 2017;65:877–884. [Google Scholar]
- Kan B, Wang M, Jing H, Xu H, Jiang X, Yan M, Liang W, Zheng H, Wan K, Liu Q, Cui B. Molecular evolution analysis and geographic investigation of severe acute respiratory syndrome coronavirus-like virus in palm civets at an animal market and on farms. J Virol. 2005;79:11892–11900. doi: 10.1128/JVI.79.18.11892-11900.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchdoerfer RN, Ward AB. Structure of the SARS-CoV nsp12 polymerase bound to nsp7 and nsp8 co-factors. Nat Commun. 2019;10:2342. doi: 10.1038/s41467-019-10280-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronenberger P, Vrijsen R, Boeyé (1991) A chloroquine induces empty capsid formation during poliovirus eclipse. J Virol 65:7008–7011 [DOI] [PMC free article] [PubMed]
- Kwiek JJ, Haystead TA, Rudolph J. Kinetic mechanism of quinine oxidoreductase 2 and its inhibition by the antimalarial quinolones. Biochemistry. 2004;43:4538–4547. doi: 10.1021/bi035923w. [DOI] [PubMed] [Google Scholar]
- Lei J, Li J, Li X, Qi X. CT imaging of the 2019 novel coronavirus (2019-nCoV) pneumonia. Radiology. 2020;295:18–18. doi: 10.1148/radiol.2020200236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Guan X, Wu P, Wang X, Zhou L, Tong Y, Ren R, Leung KS, Lau EH, Wong JY, Xing X. Early transmission dynamics in Wuhan, China, of novel coronavirus–infected pneumonia. N Engl J Med. 2020;382:1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lou Y, Liu L, Yao H, Hu X, Su J, Xu K, Luo R, Yang X, He L, Lu X, Zhao Q. Clinical outcomes and plasma concentrations of baloxavir marboxil and favipiravir in COVID-19 patients: an exploratory randomized, controlled trial. Eur J Pharm Sci. 2021;57:105631. doi: 10.1016/j.ejps.2020.105631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H. Drug treatment options for the 2019-new coronavirus (2019-nCoV) Biosci Trends. 2020;14:69–71. doi: 10.5582/bst.2020.01020. [DOI] [PubMed] [Google Scholar]
- Mina F, Rahman M, Das S, Karmakar S, Billah M (2020) Potential drug candidates underway several registered clinical trials for battling COVID19. Authorea. 10.22541/au.158949149.99681139
- Mizui T, Yamashina S, Tanida I, Takei Y, Ueno T, Sakamoto N, et al. Inhibition of hepatitis C virus replication by chloroquine targeting virusassociated autophagy. J Gastroenterol. 2010;45:195–203. doi: 10.1007/s00535-009-0132-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’hanlon R, Shaw ML (2019) Baloxavir marboxil: the new influenza drug on the market. Curr Opin Virol 35:14–18 [DOI] [PubMed]
- Parhizgar AR, Tahghighi A. Introducing new antimalarial analogues of chloroquine and amodiaquine: a narrative review. Iran J Med Sci. 2017;42:115–128. [PMC free article] [PubMed] [Google Scholar]
- Paton NI, Lee L, Xu Y, Ooi EE, Cheung YB, Archuleta S, Wong G, Smith AW. Chloroquine for influenza prevention: a randomised, double-blind, placebo controlled trial. Lancet Infect Dis. 2011;11:677–683. doi: 10.1016/S1473-3099(11)70065-2. [DOI] [PubMed] [Google Scholar]
- Phan LN, Nguyen TV, Luong QC, Nguyen TV, Nguyen HT, Le HQ, Nguyen TT, Cao TM, Pham QD. Importation and human-to-human transmission of a novel coronavirus in Vietnam. N Engl J Med. 2020;382:872–874. doi: 10.1056/NEJMc2001272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman A, Sarkar A. Risk factors for fatal Middle East respiratory syndrome coronavirus infections in Saudi Arabia: analysis of the WHO line list, 2013–2018. Am J Public Health. 2019;109:1288–1293. doi: 10.2105/AJPH.2019.305186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randolph VB, Winkler G, Stollar V. Acidotropic amines inhibit proteolytic processing of flavivirus prM protein. Virology. 1990;174:450–458. doi: 10.1016/0042-6822(90)90099-D. [DOI] [PubMed] [Google Scholar]
- Raoult D, Drancourt M, Vestris G. Bactericidal effect of doxycycline associated with lysosomotropic agents on Coxiella burnetii in P388D1 cells. Antimicrob Agents Chemother. 1990;34:1512–1514. doi: 10.1128/AAC.34.8.1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiter RJ, Ma Q, Sharma R. Treatment of Ebola and other infectious diseases: melatonin “goes viral”. Melatonin Res. 2020;3:43–57. doi: 10.32794/mr11250047. [DOI] [Google Scholar]
- Ren LL, Wang YM, Wu ZQ. Identification of a novel coronavirus causing severe pneumonia in human: a descriptive study. Chinese Med J. 2020;133:1015–1024. doi: 10.1097/CM9.0000000000000722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes A, Evans LE, Alhazzani W, Levy MM, Antonelli M, Ferrer R, Kumar A, Sevransky JE, Sprung CL, Nunnally ME, Rochwerg B. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Intensive Care Med. 2017;43:304–377. doi: 10.1007/s00134-017-4683-6. [DOI] [PubMed] [Google Scholar]
- Richardson P, Griffin I, Tucker C, Smith D, Oechsle O, Phelan A, Stebbing J. Baricitinib as potential treatment for 2019-nCoV acute respiratory disease. Lancet. 2020;395:e30–e31. doi: 10.1016/S0140-6736(20)30304-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers MC, Williams JV. Quis Custodiet Ipsos Custodes? Regulation of cell-mediated immune responses following viral lung infections. Annu Rev Virol. 2018;5:363–383. doi: 10.1146/annurev-virology-092917-043515. [DOI] [PubMed] [Google Scholar]
- Rogoveanu OC, Calina D, Cucu MG, Burada F, Docea AO, Sosoi S, Stefan E, Ioana M, Burada E. Association of cytokine gene polymorphisms with osteoarthritis susceptibility. Exp Ther Med. 2018;16:2659–2664. doi: 10.3892/etm.2018.6477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothan HA, Byrareddy SN. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J Autoimmun. 2020;109:102433. doi: 10.1016/j.jaut.2020.102433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell CD, Millar JE, Baillie JK. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. Lancet. 2020;395:473–475. doi: 10.1016/S0140-6736(20)30317-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargiacomo C, Sotgia F, Lisanti MP. COVID-19 and chronological aging: senolytics and other anti-aging drugs for the treatment or prevention of corona virus infection? Aging. 2020;12:6511. doi: 10.18632/aging.103001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar C, Mondal M, Islam MT, Martorell M, Docea AO, Maroyi A, Sharifi-Rad J, Calina D. Potential therapeutic options for COVID-19: current status, challenges, and future perspectives. Front Pharmacol. 2020;11:1428. doi: 10.3389/fphar.2020.572870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savarino A, Lucia MB, Rastrelli E, Rutella S, Golotta C, Morra E, Tamburrini E, Perno CF, Boelaert JR, Sperber K, Cauda R. Anti-HIV effects of chloroquine: inhibition of viral particle glycosylation and synergism with protease inhibitors. J Acquir Immune Defic Syndr. 1996;35:223–232. doi: 10.1097/00126334-200403010-00002. [DOI] [PubMed] [Google Scholar]
- Scavone C, Brusco S, Bertini M, Sportiello L, Rafaniello C, Zoccoli A, Berrino L, Racagni G, Rossi F. A Capuano, Current pharmacological treatments for COVID-19: What’s next? Br J Pharmacol. 2020;177:4813–4824. doi: 10.1111/bph.15072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheahan TP, Sims AC, Leist SR, Schäfer A, Won J, Brown AJ, Montgomery SA, Hogg A, Babusis D, Clarke MO, Spahn JE. Comparative therapeutic efficacy of remdesivir and combination lopinavir, ritonavir, and interferon beta against MERS-CoV. Nat Commun. 2020;11:222. doi: 10.1038/s41467-019-13940-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidiropoulou P, Docea AO, Nikolaou V, Katsarou M-S, Spandidos DA, et al. Unraveling the roles of vitamin D status and melanin during Covid-19 (review) Int J Mol Med. 2020;47:92–100. doi: 10.3892/ijmm.2020.4802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smartpatients.Com (2020) A randomized multicenter controlled clinical trial of arbidol in patients with 2019 novel coronavirus (2019-nCoV). Available at: https://www.smartpatients.com/trials/NCT04246242 (Accessed 2020 Apr)
- Steiz M, Valbracht J, Quach J, Lotz M. Gold sodium thiomalate and chloroquine inhibit cytokine production in monocytic THP-1 cells through distinct transcriptional and posttranslational mechanisms. J Clin Immunol. 2003;23:477–484. doi: 10.1023/B:JOCI.0000010424.41475.17. [DOI] [PubMed] [Google Scholar]
- Tamura DY, Moore EE, Partrick DA, Johnson JL, Offner PJ, Silliman CC. Acute hypoxemia in humans enhances the neutrophil inflammatory response. Shock. 2002;17:269–273. doi: 10.1097/00024382-200204000-00005. [DOI] [PubMed] [Google Scholar]
- Tran DH, Sugamata R, Hirose T, Suzuki S, Noguchi Y, Sugawara A, Ito F, Yamamoto T, Kawachi S, Akagawa KS, Ōmura S. Azithromycin, a 15-membered macrolide antibiotic, inhibits influenza A (H1N1) pdm09 virus infection by interfering with virus internalization process. J Antibiotics. 2019;72:759–768. doi: 10.1038/s41429-019-0204-x. [DOI] [PubMed] [Google Scholar]
- Tricou V, Minh NN, Van TP, Lee SJ, Farrar J, Wills B, Hien TT, Simmons CP. A randomized controlled trial of chloroquine for the treatment of dengue in Vietnamese adults. PLoS Negl Trop Dis. 2010;4:e785. doi: 10.1371/journal.pntd.0000785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsiang H, Superti F. Ammonium chloride and chloroquine inhibit rabies virus infection in neuroblastoma cells. Arch Virol. 1984;81:377–382. doi: 10.1007/BF01310010. [DOI] [PubMed] [Google Scholar]
- Varki A. Sialic acids as ligands in recognition phenomena. FASEB J. 1997;11:248–255. doi: 10.1096/fasebj.11.4.9068613. [DOI] [PubMed] [Google Scholar]
- Vincent MJ, Bergeron E, Benjannet S, Erickson BR, Rollin PE, Ksiazek TG. Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virol J. 2005;2:69. doi: 10.1186/1743-422X-2-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, Wang B, Xiang H, Cheng Z, Xiong Y, Zhao Y. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Cao R, Zhang L, Yang X, Liu J, Xu M, Shi Z, Hu Z, Zhong W, Xiao G. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang RR, Yang QH, Luo RH, Peng YM, Dai SX, Zhang XJ, Chen H, Cui XQ, Liu YJ, Huang JF, Chang JB. Azvudine, a novel nucleoside reverse transcriptase inhibitor showed good drug combination features and better inhibition on drug-resistant strains than lamivudine in vitro. PLoS ONE. 2021;9:e105617. doi: 10.1371/journal.pone.0105617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Tang J, Wei F. Updated understanding of the outbreak of 2019 novel coronavirus (2019-nCoV) in Wuhan, China. J Med Virol. 2020;92:441–447. doi: 10.1002/jmv.25689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Zhang D, Du G, Du R, Zhao J, Jin Y, Fu S, Gao L, Cheng Z, Lu Q, Hu Y. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020;395:1569–1578. doi: 10.1016/S0140-6736(20)31022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO (2020a) Clinical Management of Severe Acute Respiratory Infection When Novel Coronavirus (nCoV) Infection Is Suspected: Interim Guidance. https://www.who.int/publicationsdetail/clinical-management-of-severe-acute-respiratoryinfection-when-novel-coronavirus-(ncov)-infection-is-suspected. WHO website. Updated March 13, 2020a. Accessed Mar 23, 2020b
- WHO (2020b) Clinical management of severe acute respiratory infection when novel coronavirus (nCoV) infection is suspected. https://www.who.int/publications-detail/clinical-management-of-severe acute respiratory-infection-when-novelcoronavirus-(ncov)-infection-is-suspected. Published January 28, 2020b. Accessed Mar 19 2020c
- WHO (2020c) Clinical management of severe acute respiratory infection when novel coronavirus (nCoV) infection is suspected, https://www.who.int/publications-detail/clinical-managementof-severe-acute-respiratory-infection-whennovel-coronavirus-(ncov)-infection-is-suspected. Accessed Jan 28 2020a
- Wu X, Ji H, Wang Y, Gu C, Gu W, Hu L, Zhu L (2019) Melatonin alleviates radiation-induced lung injury via regulation of miR 30e/NLRP3 axis. Oxid Med Cell Longev 2019 2019:4087298 [DOI] [PMC free article] [PubMed]
- Zhang Q, Wang Y, Qi C, Shen L, Li J (2020a) Clinical trial analysis of 2019-nCoV therapy registered in China. J Med Virol 92:540–545 [DOI] [PMC free article] [PubMed]
- Zhang R, Wang X, Ni L, Di X, Ma B, Niu S, Liu C, Reiter RJ. COVID-19: melatonin as a potential adjuvant treatment. Life Sci. 2018;250:117583. doi: 10.1016/j.lfs.2020.117583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Zhao Y, Zhang F, Wang Q, Li T, Liu Z, Wang J, Qin Y, Zhang X, Yan X, Zeng X. The use of anti-inflammatory drugs in the treatment of people with severe coronavirus disease 2019 (COVID-19): the experience of clinical immunologists from China. Clin Immunol. 2020;214:108393. doi: 10.1016/j.clim.2020.108393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao S, Lin Q, Ran J, Musa SS, Yang G, Wang W, Lou Y, Gao D, Yang L, He D, Wang MH. Preliminary estimation of the basic reproduction number of novel coronavirus (2019-nCoV) in China, from 2019 to 2020: a data-driven analysis in the early phase of the outbreak. Int J Infect Dis. 2020;92:214–217. doi: 10.1016/j.ijid.2020.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong N, Zheng B, Li Y, Poon L, Xie Z, Chan K, Li PH, Tan SY, Chang Q, Xie JP, Liu XQ. Epidemiology and cause of severe acute respiratory syndrome (SARS) in Guangdong, People’s Republic of China, in February, 2003. Lancet. 2003;362:1353–1358. doi: 10.1016/S0140-6736(03)14630-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, Si HR, Zhu Y, Li B, Huang CL, Chen HD. Discovery of a novel coronavirus associated with the recent pneumonia outbreak in humans and its potential bat origin. BioRxiv. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou L, Ruan F, Huang M, Liang L, Huang H, Hong Z, Yu J, Kang M, Song Y, Xia J, Guo Q. SARS-CoV-2 viral load in upper respiratory specimens of infected patients. N Engl J Med. 2020;382:1177–1179. doi: 10.1056/NEJMc2001737. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.