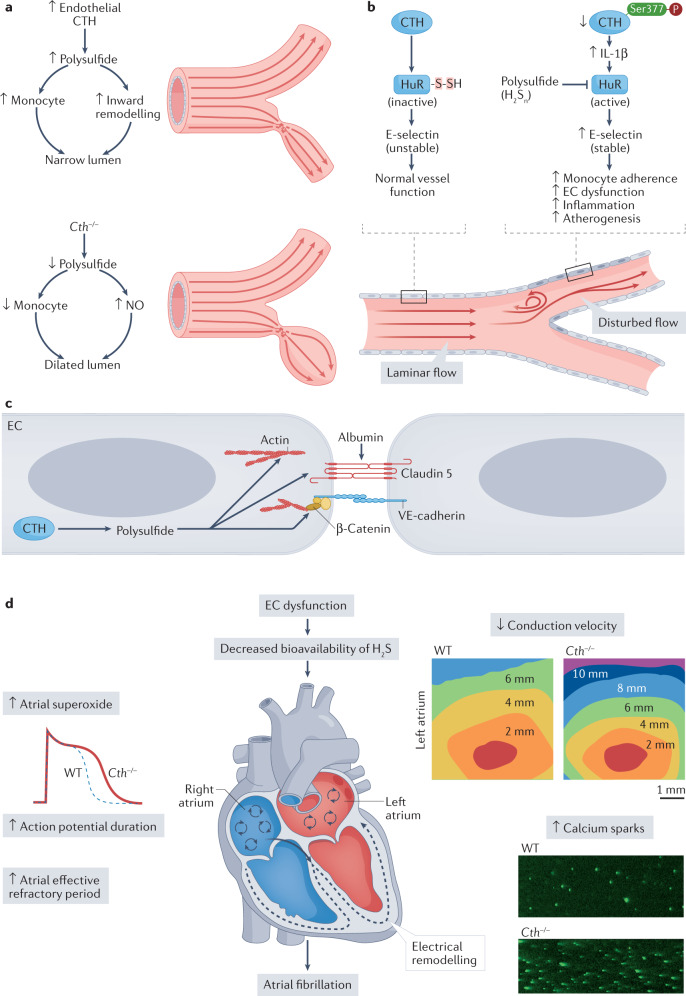
Fig. 3. Sulfide regulation of cardiovascular responses involving CTH expression and function.
a | Cystathionine γ-lyase (CTH) expression and sulfane sulfur production are increased by disturbed blood flow in conduit vessels, causing increased macrophage recruitment to these areas, leading to flow-induced vascular remodelling. In Cth−/− mice, sulfane sulfur levels in response to partial carotid artery ligation are reduced, leading to defective inward remodelling and a dilated vascular phenotype, which results from elevated nitric oxide (NO) bioavailability. b | In regions of laminar blood flow, CTH-derived polysulfide inactivates human antigen R (HuR) via S-sulfhydration (HuR-S-SH), thereby attenuating E-selectin expression, which regulates vascular inflammation and atherogenesis. In regions of disturbed blood flow, defective CTH or polysulfide leads to HuR activation and subsequent E-selectin stability, which induces endothelial cell (EC) dysfunction and atherogenesis. c | Regulation of endothelial permeability by CTH-derived sulfur species increases endothelial solute permeability and leads to disruption of the endothelial junction proteins claudin 5 and vascular endothelial (VE)-cadherin, together with increased actin stress fibre formation. d | Hydrogen sulfide (H2S) modulates cardiac ion channels both directly and indirectly, leading to electrical remodelling. Reduced CTH-derived sulfide bioavailability (for example, owing to EC dysfunction or in Cth−/− mice) increases atrial superoxide levels and the frequency of atrial cell calcium sparks, slows atrial conduction velocity and prolongs both the action potential duration and atrial effective refractory period, all of which contribute to the development of atrial fibrillation. WT, wild-type.