Abstract
Purpose:
Ripretinib is a switch-control tyrosine kinase inhibitor that broadly inhibits KIT and PDGFRA kinase signaling. Ripretinib showed preliminary efficacy in patients with advanced gastrointestinal stromal tumor (GIST) in a phase 1 study across a range of doses. Results were confirmed in the phase 3 INVICTUS study, and ripretinib 150 mg once daily (QD) was subsequently approved as a ≥fourth-line therapy. Here, we report the phase 1 study results of intrapatient dose escalation (IPDE) in patients with GIST treated across second, third, and later lines of therapy.
Methods:
Patients with advanced GIST who experienced disease progression (PD) at ripretinib 150 mg QD could dose escalate to 150 mg twice daily (BID). PFS1 was calculated from date of first dose of ripretinib 150 mg QD to PD (as per RECIST 1.1); PFS2 was from date of IPDE (150 mg BID) to PD or death. Treatment-emergent adverse events (TEAEs) were summarized by dosing periods and compared descriptively.
Results:
Of 142 patients with GIST receiving ripretinib 150 mg QD, 67 underwent IPDE. IPDE provided benefit across all lines of therapy; mPFS2 was 5.6, 3.3, and 4.6 months for patients on second-, third-, and ≥fourth-line therapy, respectively. A partial metabolic response following IPDE was demonstrated in 13 of 37 patients with available PET scans. TEAEs reported at both doses were similar.
Conclusion:
Ripretinib IPDE after PD provided continued clinical benefit in advanced GIST across second, third, and later lines of therapy with a similar safety profile to that observed with the QD regimen.
Keywords: Gastrointestinal stromal tumors, ripretinib, pharmacology, disease progression, progression-free survival
Introduction
Most gastrointestinal stromal tumors (GIST) harbor an activating mutation in either KIT or platelet-derived growth factor receptor α (PDGFRA) tyrosine kinases.1–3 Treatment of advanced GIST improved greatly with approval of oral tyrosine kinase inhibitors (TKIs). Five TKIs are approved for GIST in the US—imatinib (adjuvant and first-line therapy), sunitinib (second-line therapy), regorafenib (third-line therapy), ripretinib (fourth-line therapy), and avapritinib (PDGFRA exon 18 mutant GIST).4–7 While these TKIs improved the outcomes of patients with GIST, disease progression still occurs, with progression-free survival (PFS) being typically shorter following first-line treatment. Disease progression is largely due to the development of secondary mutations in KIT or PDGFRA,8 which can result in complex intra- and inter-tumor heterogeneity.8,9 Thus, there is a need for therapeutic options in advanced GIST that are effective against a broad spectrum of KIT and PDGFRA mutations and provide clinical benefit beyond disease progression.
Ripretinib, approved for use based on the results of the phase 3 INVICTUS trial,10 is indicated in the treatment of adult patients with advanced GIST who have received prior treatment with 3 or more kinase inhibitors, including imatinib.5,11,12 Ripretinib, an oral switch-control TKI, has a unique dual mechanism of action that regulates the kinase switch pocket and activation loop.13 This novel mechanism of action provides broad inhibition of KIT or PDGFRA kinase activity, including wild type KIT or PDGFRA and multiple KIT and PDGFRA mutations.13 In the primary report of the phase 1 trial, ripretinib demonstrated promising efficacy and had a favorable safety profile in patients with advanced GIST treated across multiple lines of therapy.14 While no maximum-tolerated dose (MTD) was reached, the recommended dose of ripretinib was established as 150 mg once daily (QD) based on safety, pharmacokinetic, and pharmacodynamic findings.14
Intrapatient dose escalation (IPDE), as an alternative therapeutic option following disease progression while on an approved TKI, was previously demonstrated in patients receiving imatinib who, following progression, were allowed to cross over to a higher dose with benefit to a subset of patients.15 A similar approach was explored in the ripretinib phase 1 study; upon disease progression with ripretinib 150 mg QD, patients could dose escalate to ripretinib 150 mg twice daily (BID). Here, we report the efficacy, pharmacokinetics (PK), and from the phase 1 study.
Materials and Methods
Study design and treatment
Detailed methods of the phase I study (NCT02571036) that included dose escalation and expansion phase, have been previously described.14 The escalation phase evaluated increasing doses of ripretinib administered in 28-day cycles in patients with advanced malignancies.14 This escalation phase resulted in the recommended dose of 150 mg QD.14 In the expansion phase, patients were enrolled into disease-specific cohorts, including the KIT/PDGFRA mutant GIST cohort, and started on the recommended 150 mg QD dose of ripretinib.14 The GIST cohort consisted of patients treated with 1 (second-line therapy), 2 (third-line therapy), or ≥3 (fourth-line or greater therapy) prior anticancer therapies.14 Patients who progressed on ripretinib 150 mg QD, as determined by Response Evaluation Criteria In Solid Tumors (RECIST) v1.1 and based on local radiology review, were given the option to escalate to 150 mg BID.
This study was conducted in accordance with the Declaration of Helsinki and the International Council for Harmonization Guidelines for Good Clinical Practice. All patients provided written informed consent to participate in the study. Prior to the start of and throughout the study, the protocol, protocol amendments, and informed consent documents were approved by each site’s institutional review board and the appropriate regulatory authorities.
Patient population
Eligible patients included those ≥18 years of age with histologically confirmed advanced GIST.14 Patients must have had ≥1 measurable lesion, according to RECIST version 1.1. For inclusion in the trial, patients with GIST were required to have a KIT or PDGFRA mutation and must have progressed on, or had an intolerability to, at least 1 line of systemic TKIs. Baseline characteristics, including age, sex, Eastern Cooperative Oncology Group (ECOG) status, and mutation status, were recorded. Full eligibility criteria are listed in Supplementary Data.
Efficacy assessments
The primary efficacy outcome was PFS per RECIST v1.1 based on local radiology review. PFS1 was calculated from the date of first dose of ripretinib 150 mg QD to disease progression. PFS2 was defined as PFS on ripretinib 150 mg BID from the date of escalation to progression or death. An exploratory assessment of metabolic tumor response by 18F-fluorodeoxyglucose positron emission tomography (FDG-PET), according to European Organisation for Research and Treatment of Cancer (EORTC) criteria, was also conducted during the PFS2 period following the patient’s initial progression.18 PFS2 baseline positron emission tomography (PET) scans were taken within 10 days prior to IPDE; a follow-up scan was taken 17–31 days after IPDE. PET scans were assessed by a central reviewer.
Pharmacokinetic assessments
The PK of ripretinib and its active metabolite DP-5439 were assessed. PK sampling was conducted predose on day 15 during cycle 1 and the first day of each subsequent cycle, and at the final study visit. PK analyses are presented as geometric mean predose steady state trough plasma concentration of ripretinib and active metabolite DP-5439 at the 150 mg QD and 150 mg BID dose. Steady state was defined as when the regimen was administered >50% of the time for the specified interval (ie, 150 mg BID dose was administered most of the time during the IPDE period) and regimens for which the average number of days administered ≥5 days (ie, patient is conceivably dosed to steady state for each observed trough concentration). Plasma samples were analyzed for ripretinib and DP-5439 concentrations using a validated high-performance liquid chromatography tandem mass spectrometric method.
Safety outcomes
Treatment-emergent adverse events (TEAEs) were defined as any adverse event (AE) that occurred after administration of the first dose of study drug and through 30 days after the last dose of study drug, any event that was considered drug-related, regardless of the start date of the event, or any event that was present at baseline but worsened in severity, or any event that was subsequently considered drug-related by the investigator. AEs were coded with MedDRA dictionary v21.1. AEs are presented for patients with GIST who received ripretinib 150 mg QD and dose escalated to 150 mg BID.
Statistical analyses
Analyses were conducted by line of therapy (second, third, and fourth or greater). Descriptive statistics were used to summarize continuous variables and discrete variables were summarized using frequencies and percentages. For PFS, the Kaplan-Meier method was used to obtain nonparametric estimates, the associated 2-sided 95% confidence intervals (CIs) for the median survival time, the 25th and 75th percentiles, and survival probability functions.
Results
Patients
As of May 8, 2020 (data cutoff), of 142 enrolled patients with advanced GIST receiving ripretinib 150 mg QD, 67 patients dose escalated to ripretinib 150 mg BID after disease progression (Supplemental Figure 1). The IPDE population (n = 67) included 10 patients on second-line therapy, 17 patients on third-line therapy, and 40 patients on fourth-line or greater therapy. Overall baseline patient characteristics are listed in Table 1.
Table 1.
Baseline characteristics of the IPDE population
| Characteristics | 2nd line (n = 10) | 3rd line (n = 17) | ≥4th line (n = 40) | Total (N = 67) |
|---|---|---|---|---|
| Age at informed consent (years) | ||||
| Mean (SD) | 59.6 (13.57) | 64.6 (8.66) | 59.9 (10.03) | 61.1 (10.35) |
| Median | 60.0 | 64.0 | 59.0 | 60.0 |
| Range | 32, 80 | 51, 82 | 39, 87 | 32, 87 |
| Age category (years) | ||||
| ≥18–≤64 | 6 (60) | 9 (53) | 30 (75) | 45 (67) |
| ≥65 | 4 (40) | 8 (47) | 10 (25) | 22 (33) |
| Sex | ||||
| Male | 3 (30) | 10 (59) | 30 (75) | 43 (64) |
| Female | 7 (70) | 7 (41) | 10 (25) | 24 (36) |
| ECOG status | ||||
| 0 | 8 (80) | 9 (53) | 19 (48) | 36 (54) |
| 1 | 2 (20) | 8 (47) | 20 (50) | 30 (45) |
| 2 | 0 | 0 | 1 (3) | 1 (2) |
| Primary Mutations | ||||
| KIT exon 11 | 8 (80) | 12 (71) | 28 (70) | 48 (72) |
| KIT exon 9 | 1 (10) | 5 (29) | 8 (20) | 14 (21) |
| KIT other exons | 0 | 0 | 2 (5) | 2 (3) |
| PDGFRA (exon 18, non-D842V) | 1 (10) | 0 | 2 (5) | 3 (5) |
Data presented as n (%) unless otherwise indicated. Percentages were rounded to the nearest whole number.
ECOG, Eastern Cooperative Oncology Group; IPDE, intrapatient dose escalation; PDGFRA, platelet-derived growth factor receptor alpha; SD, standard deviation.
Efficacy
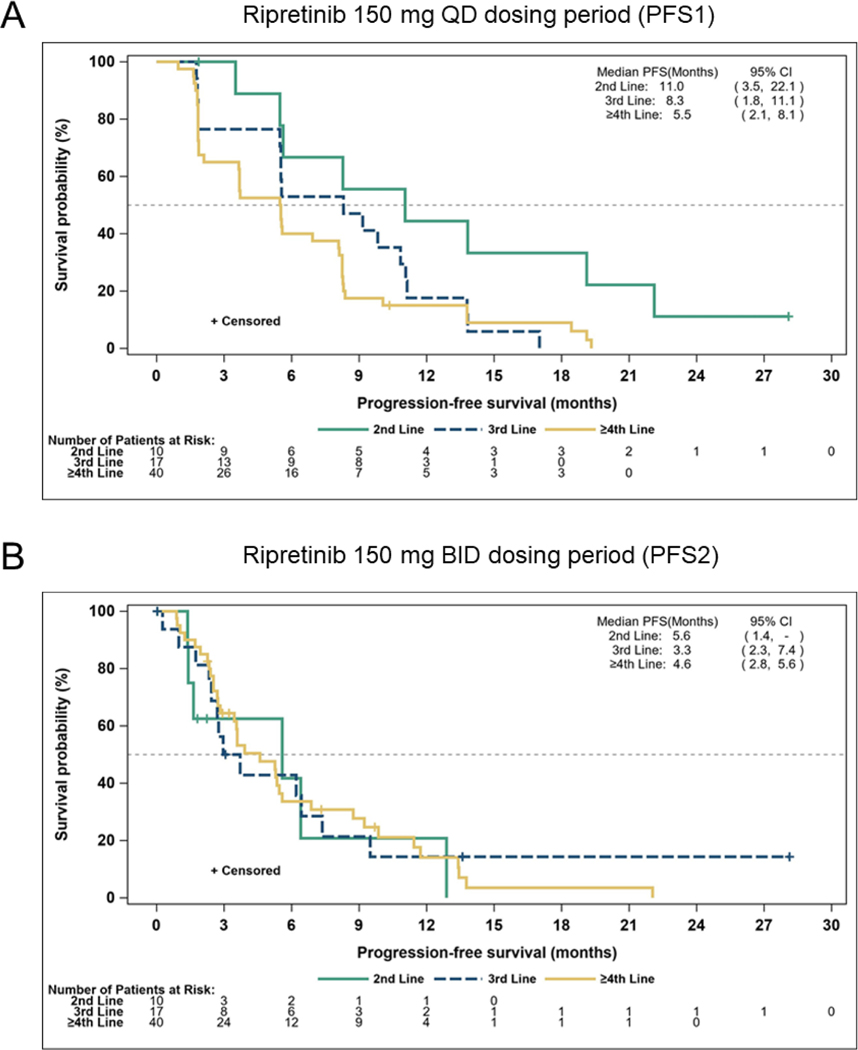
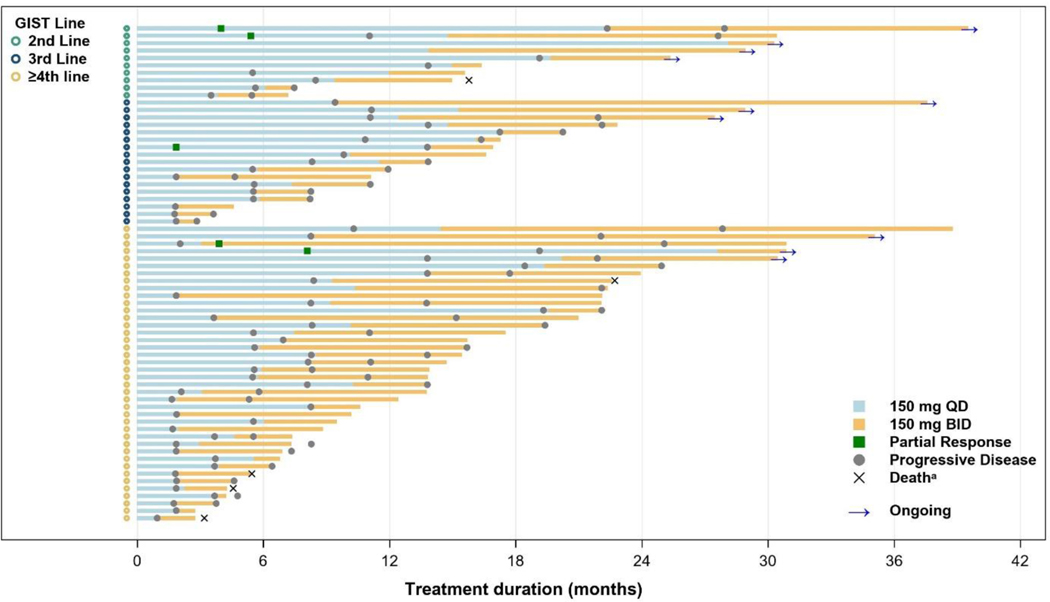
In the IPDE population, the median PFS1 (mPFS1; 150 mg QD) was 11.0 months (95% CI, 3.5, 22.1 months) for patients on second-line therapy, 8.3 months (95% CI, 1.8, 11.1 months) for third-line therapy, and 5.5 months (95% CI, 2.1, 8.1 months) for fourth-line or greater therapy (Figure 1A). Following IPDE, the median PFS2 (mPFS2) was 5.6 months (95% CI, 1.4, not estimable) for patients on second-line therapy, 3.3 months (95% CI, 2.3, 7.4 months) for third-line therapy, and 4.6 months (95% CI, 2.8, 5.6 months) for fourth-line or greater therapy (Figure 1B). The ratio of mPFS2/mPFS1 in patients with GIST was 51%, 40%, and 84% for second-line, third-line, and fourth-line or greater therapy, respectively. Treatment duration for both dosing periods is shown in Figure 2. Within the IPDE population, across all lines of therapy, 14 patients with advanced GIST were on treatment for 2 years or longer. At time of data cutoff, 10 patients were continuing treatment, including 4 who showed disease progression at higher dose ripretinib but continued treatment for clinical benefit.
Figure 1.
Kaplan-Meier plot of progression-free survival by line of therapy in patients with GIST who underwent intrapatient dose escalation
BID, twice daily; CI, confidence interval; GIST, gastrointestinal stromal tumor; PFS, progression-free survival; QD, once daily; -, not estimable.
Figure 2.
Total duration of treatment in the GIST intrapatient dose escalation population
a Deaths noted were those counted as PFS events.
BID, twice daily; GIST, gastrointestinal stromal tumor; PFS, progression-free survival; QD, once daily.
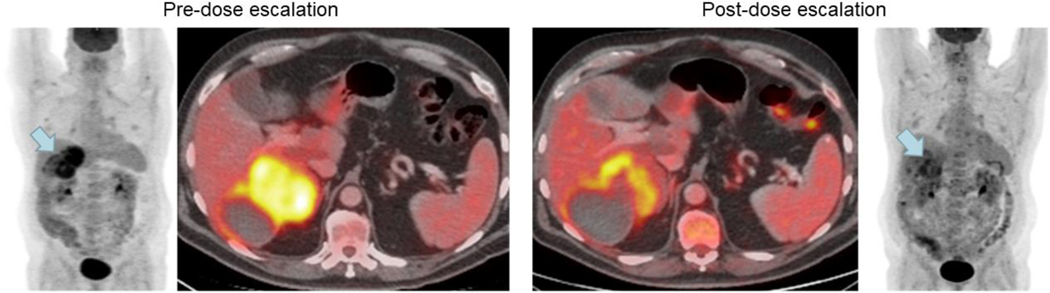
A total of 37 patients underwent pre/post-escalation PET scans within the window. Assessment of tumor metabolic response per EORTC criteria in patients with GIST that underwent IPDE (n = 37) was performed. A total of 13 (35.1%) patients, across all lines of therapy, demonstrated partial metabolic response and 19 (51.4%) showed metabolic stable disease following dose escalation to ripretinib 150 mg BID compared to baseline. A summary of best overall response by line of therapy is shown in Supplemental Table 2. Radiological images of a partial metabolic response (−33.7% change in standardized uptake values) pre- and post-dose escalation from a patient in this study are shown in Figure 3.
Figure 3.
Radiological images of a partial metabolic response in a patient with GIST who underwent ripretinib intrapatient dose escalation
GIST, gastrointestinal stromal tumor.
Pharmacokinetics
PK analysis (n = 60) revealed that subsequent dose escalation from 150 mg QD to BID resulted in an approximately 2-fold increase in the steady state trough concentration of ripretinib and its active metabolite DP-5439. Patients who were missing or had invalid steady state trough samples at either regimen (QD or IPDE) were excluded from the PK analysis. Geometric mean trough concentrations for ripretinib increased from 323 ng/mL at 150 mg QD to 676 ng/mL at 150 mg BID, while DP-5439 increased from 721 ng/mL to 1270 ng/mL post-dose escalation.
Safety
The AE profile was similar during both dosing periods in patients with advanced GIST. TEAEs that occurred in >10% of the IPDE population during the IPDE period are listed in Table 2, with the corresponding TEAE frequencies during the QD dosing period provided for reference. Of note, only TEAEs that were new or worsened during the IPDE period are listed for that time period. Two TEAEs were notably higher in the IPDE period compared to the QD period, anemia (22.4% vs 4.5%) and dyspnea (13.4 vs 7.5%). No new TEAEs (>10%) were observed in the IPDE period, although two Grade 3/4 AEs (>5% of patients)—abdominal pain (10.4%) and anemia (6%)—were observed in the IPDE period only. Of 67 IPDE patients, 32 (47.8%) developed a Grade 3/4 TEAE during the QD dosing period and 42 (62.7%) developed a Grade 3/4 TEAE during the IPDE period. Twelve (17.9%) patients experienced a TEAE that led to death, none of which were considered related to study drug.
Table 2.
TEAEs occurring in >10% of patients with GIST in the 150 mg IPDE period
| Preferred term, n (%) | Ripretinib 150 mg QD period (n = 67) | Ripretinib 150 mg IPDE perioda (n = 67) | ||
|---|---|---|---|---|
| All grades | Grade 3/4 | All grades | Grade 3/4 | |
| Diarrhea | 13 (19.4) | 1 (1.5) | 19 (28.4) | 1 (1.5) |
| Abdominal pain | 15 (22.4) | 0 | 18 (26.9) | 7 (10.4) |
| Nausea | 24 (35.8) | 0 | 17 (25.4) | 0 |
| Decreased appetite | 11 (16.4) | 0 | 16 (23.9) | 1 (1.5) |
| Anemia | 3 (4.5) | 0 | 15 (22.4) | 4 (6.0) |
| Fatigue | 23 (34.3) | 0 | 14 (20.9) | 2 (3.0) |
| PPES | 24 (35.8) | 0 | 12 (17.9) | 0 |
| Alopecia | 41 (61.2) | 0 | 11 (16.4) | 0 |
| Vomiting | 9 (13.4) | 0 | 11 (16.4) | 0 |
| Weight decreased | 19 (28.4) | 0 | 11 (16.4) | 0 |
| Muscle spasms | 19 (28.4) | 0 | 10 (14.9) | 0 |
| Dyspnea | 5 (7.5) | 0 | 9 (13.4) | 2 (3.0) |
| Back pain | 10 (14.9) | 0 | 7 (10.4) | 0 |
| Headache | 8 (11.9) | 0 | 7 (10.4) | 1 (1.5) |
| Myalgia | 33 (49.3) | 0 | 7 (10.4) | 0 |
| Oedema peripheral | 5 (7.5) | 0 | 7 (10.4) | 1 (1.5) |
| Rash | 13 (19.4) | 0 | 7 (10.4) | 0 |
Only includes TEAEs that are new or worsening.
GIST, gastrointestinal stromal tumor; IPDE, intrapatient dose escalation; PPES, palmar-plantar erythrodysesthesia syndrome; QD, once daily; TEAE, treatment-emergent adverse event.
Similar numbers of patients underwent dose reduction/interruptions due to TEAEs in both dosing periods. A summary of dose modifications that occurred during both dosing periods is presented in Table 3. After dose escalation to ripretinib 150 mg BID, 10 (15%) patients experienced TEAEs that led to treatment discontinuation (Table 3). We reviewed AEs that led to dose interruption/reductions and did not observe a trend toward any specific AE. Events that led to treatment discontinuation in 10 patients are presented in Supplemental Table 1. Three of these 10 events—cardiac failure, cardiac myopathy, and ejection fraction decreased—were considered drug-related.
Table 3.
Dose modifications occurring in patients with GIST in the 150 mg IPDE population
| Parameters, n (%) | Ripretinib 150 mg QD period (n = 67) | Ripretinib 150 mg IPDE period (n = 67) |
|---|---|---|
| Any TEAE leading to dose interruption | 28 (41.8) | 30 (44.8) |
| Any TEAE leading to dose reduction | 4 (6.0) | 6 (9.0) |
| Any TEAE leading to treatment discontinuation | N/A | 10 (14.9) |
GIST, gastrointestinal stromal tumor; IPDE, intrapatient dose escalation; N/A, not applicable; QD, once daily; TEAE, treatment-emergent adverse event.
Discussion
In the primary report of this phase 1 trial, ripretinib demonstrated promising efficacy at a range of doses in patients with advanced GIST.14 The MTD was not reached, and initial PK analysis determined peak plasma concentration (mean Cmax [coefficient of variation %]) following a single dose of 150 mg ripretinib on cycle 1 day 1 to be 502 ng/mL (56.8%) and exposure (AUC0–24h) was 6634 ng x h/mL (59.8%).14 Preclinical pharmacology studies predicted ripretinib 150 mg to be effective, and thus, combined with the phase 1 results, 150 mg QD was established as the recommended dose.14
The current study aimed to determine the efficacy, PK, and safety of ripretinib dose escalation following disease progression. Efficacy results of IPDE in patients with GIST demonstrate promising activity across all lines of therapy tested. The mPFS1 by line of therapy in the IPDE population closely matches the overall mPFS for all GIST patients enrolled in the phase 1 study.14 Following disease progression, dose escalation to ripretinib 150 mg BID resulted in additional PFS beyond the mPFS1 in these patients by 51%, 40%, and 84% for second-, third-, and fourth-line or greater therapy, respectively. Additionally, an exploratory analysis of metabolic response showed 35.1% of patients had a partial metabolic response upon IPDE.
In general, ripretinib 150 mg BID had an acceptable safety profile similar to 150 mg QD, and IPDE resulted in an approximately 2-fold increase in the steady state trough concentration of ripretinib. No new TEAEs were observed with the higher dose. TEAEs leading to dose interruption or dose reduction are comparable during the 150 mg QD period and IPDE period. Ten of 67 (15%) patients discontinued treatment due to TEAEs during the IPDE period, compared to 12 of 142 (8.5%) patients who discontinued treatment due to AEs on ripretinib 150 mg QD.14 Three cardiac events were observed and considered related to study drug. Cardiac dysfunction is a safety warning for the 150 mg QD ripretinib regimen and label precautions indicate that ejection fraction by echocardiogram or multigated acquisition scan should be assessed prior to initiating ripretinib and during treatment, as clinically indicated.5
An IPDE study with TKIs in the treatment of GIST was previously done in a large clinical trial of patients with advanced GIST on imatinib as a first-line therapy. After disease progression on imatinib 400 mg QD, 133 patients crossed over to imatinib 400 mg BID, resulting in a mPFS of 81 days. However, the benefit was limited to patients with KIT exon 9 primary mutations.15 Although dose escalation was concluded to be well tolerated, a significant increase in reports of anemia and fatigue were noted. At 6 months post-dose escalation, 17% of patients required a dose reduction and a further 51% discontinued treatment largely as a result of disease progression.15 While dose escalation for imatinib is recommended in certain clinical scenarios, specifically, KIT exon 9 mutations, this benefit has not been demonstrated for the other TKIs.16
A limitation of this study is that not all patients who experienced progressive disease received 150 mg BID dose (Supplemental Figure 1). The decision of dose escalation was made by individual investigators based on the patient’s best interest. Another limitation is the relatively small sample size across multiple lines of therapy and did not allow for analyses to be stratified by mutational status or to determine whether increased drug exposure led to increased PFS.
Conclusions
In conclusion, ripretinib dose escalation (150 mg QD to 150 mg BID) following disease progression was well tolerated and provided additional clinical benefit for patients with advanced GIST. This promising benefit was demonstrated for patients with GIST receiving second-, third-, and fourth-line-or-greater therapy. Ripretinib IPDE may be a clinically meaningful and well-tolerated strategy for patients with advanced GIST who progress on ripretinib 150 mg QD.
Supplementary Material
Highlights.
Treatment options are limited for advanced gastrointestinal stromal tumor (GIST)
Ripretinib is a novel oral switch-control tyrosine kinase inhibitor
Ripretinib is approved for patients with advanced GIST in the 4th line and beyond
Ripretinib dose escalation at progression provides clinical benefit in advanced GIST
Acknowledgments
We would like to thank the patients, their families and caregivers, the investigators, and the investigational site staff. This study was sponsored by Deciphera Pharmaceuticals, LLC, Waltham, MA, USA. Medical writing support was provided by Helen Rodgers, PhD, of AlphaBioCom, LLC, King of Prussia, PA, USA, and was funded by Deciphera Pharmaceuticals, LLC. MCH received partial salary support from a VA Merit Award Grant (2I01BX000338-05).
Funding:
This study was sponsored by Deciphera Pharmaceuticals, LLC, Waltham, MA, USA.
Conflict of interest statement
SG serves in an advisory/consultancy role for AstraZeneca, Bayer, Blueprint Medicines, Daiichi Sankyo, Deciphera Pharmaceuticals, Eli Lilly, and Exelixis; has a leadership role in Alliance Foundation; receives licensing royalties from Wolters Kluwer Health; is a shareholder/stockholder of Abbott Labs and Allergan; and her institution receives research support from Bayer, Blueprint Medicines, Deciphera Pharmaceuticals, Novartis, and Pfizer. PC serves in an advisory role for Deciphera Pharmaceuticals, Exelixis, and Zailab; has received grant funding from Deciphera Pharmaceuticals, Exelixis, Novartis, and Array; and has licensing royalties and owns stock in ORIC. MCH serves in a consultancy role for Novartis, Deciphera Pharmaceuticals, Blueprint Medicines; receives royalties from Novartis; receives grant funding from Blueprint Medicines and Deciphera Pharmaceuticals; and has received travel, accommodations, and expenses from Blueprint Medicines, and Deciphera Pharmaceuticals. MvM serves in an advisory/consultancy role for Deciphera Pharmaceuticals, Blueprint Medicines, and Exelexis; has received travel/accommodation expenses from Deciphera Pharmaceuticals, and NCCN; and her institution has received funding from Arog, ASCO, Blueprint Medicines, Deciphera Pharmaceuticals, Garadalis, GenMab, Novartis, and Solarius. RLJ has received honoraria and serves in an advisory role for Adapimmune Therapeutics, Athenex, Bayer, Boehringer Ingelheim, Blueprint Medicines, Clinigen Group, Daiichi Sankyo, Deciphera Pharmaceuticals, Eisai, Epizyme, Immunedesign, Eli Lilly, Merck, Pharmamar, UptoDate; and serves in an advisory role for Boehringer Ingelheim, and Tracon; and has received funding from MSD. KG serves in an advisory role for Daiichi Sankyo and Foundation Medicine and her institution has received grant funding from Deciphera Pharmaceuticals. JT serves in an advisory/consultancy role for Blueprint Medicines, Deciphera Pharmaceuticals, Daiichi Sankyo, Epizyme, and Agios; and his institution has received grant funding from Blueprint Medicines, Deciphera Pharmaceuticals, Plexxicon, Advanchen, and Agios. HG institution has received grant funding from Daiichi Sankyo, Five Prime, Novartis, Deciphera Pharmaceuticals, Eli Lilly, Roche, Eisai, Debio, Boehringer Ingelheim, Pfizer, Amgen, and TEVA. AAR institution has received grant funding from Deciphera Pharmaceuticals. MSG serves in an advisory/consultancy role for Deciphera Pharmaceuticals, Tracon, ImaginAB, Imaging Endpoints, Daiichi Sankyo, Agenus, Salarius, RedHill Biopharma; has a leadership role in CareMission; his institution has received grant funding from Medimmune, Merck, Bristol-Meyers Squibb, Amgen, Tesaro, Beigene, AbbVie, Aeglea, Agenus, Arcus, Astex, Blueprint Medicines, Calithera, CellDex, Corcept, Clovis, Eli Lilly, Endocyte, Five Prime, Genocea, Neon, Plexxicon, Revolution Medicine, Seattle Genetics, Serono, SynDevRx, Tolero, Tracon, Deciphera Pharmaceuticals, Salarius, Roche/Genentech, Syndax, RedHill Biopharma, FujiFilm Pharma, Veru, Daiichi Sankyo, and ImaginAB; and owns stock in Medelis. NS serves in an advisory role for Deciphera Pharmaceuticals, Bayer, and Blueprint Medicines; has stocks in Pfizer and has received grant funding from Deciphera Pharmaceuticals, Ascentage, Daiichi Sanyko, AstraZeneca, GSK and Karyopharm. JJ is employed by Deciphera Pharmaceuticals. JM is employed by Deciphera Pharmaceuticals and owns stock in Deciphera Pharmaceuticals. KS is employed by Deciphera Pharmaceuticals, and owns stock in Alnylam, Immunogen, Karyopharm, Deciphera Pharmaceuticals, Spectrum, AstraZeneca and Alberio. YS is employed by Deciphera Pharmaceuticals. RR-S is employed by Deciphera Pharmaceuticals, and owns stock in Deciphera Pharmaceuticals, and Immunogen. FJ serves in an advisory role for Asana, Baush Health, Cardiff Oncology, Deciphera, Guardant Health, Ideaya, IFM Therapeutics, Immunomet, Illumina, Jazz Pharmaceuticals, Novartis, PureTech Health, Sotio, and Synlogic; has stocks in Cardiff Oncology and his institution receives funding from Agios, Asana, Astellas, Astex, Bayer, Bicara, BioMed Valley Discoveries, Bioxcel, Bristol-Myers Squibb, Deciphera, FujiFilm Pharma, Genentech, Ideaya, JS Innopharm, Eli Lilly, Merck, Novartis, Novellus, Plexxikon, Proximagen, Sanofi, Sotio, SpringBank Pharmaceuticals, SQZ Biotechnologies, Synlogic, Synthorx, and Symphogen.
Footnotes
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Corless CL, Schroeder A, Griffith D, et al. PDGFRA mutations in gastrointestinal stromal tumors: frequency, spectrum and in vitro sensitivity to imatinib. J Clin Oncol. 2005;23(23):5357–5364. [DOI] [PubMed] [Google Scholar]
- 2.Martin-Broto J, Martinez-Marin V, Serrano C, et al. Gastrointestinal stromal tumors (GISTs): SEAP-SEOM consensus on pathologic and molecular diagnosis. Clin Transl Oncol. 2017;19(5):536–545. [DOI] [PubMed] [Google Scholar]
- 3.Szucs Z, Thway K, Fisher C, et al. Molecular subtypes of gastrointestinal stromal tumors and their prognostic and therapeutic implications. Future Oncol. 2017;13(1):93–107. [DOI] [PubMed] [Google Scholar]
- 4.<au>Gleevec</au>. Prescribing Information. In. East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2020. [Google Scholar]
- 5.Qinlock. Prescribing information. In. Waltham, MA: Deciphera Pharmaceuticals, LLC; 2020. [Google Scholar]
- 6.Stivarga. Prescribing Information. In. Whippany, NJ: Bayer HealthCare Pharmaceuticals Inc.,; 2020. [Google Scholar]
- 7.Sutent. Prescribing Information. In. New York, NY: Pfizer Labs; 2020. [Google Scholar]
- 8.Serrano C, Marino-Enriquez A, Tao DL, et al. Complementary activity of tyrosine kinase inhibitors against secondary kit mutations in imatinib-resistant gastrointestinal stromal tumours. Br J Cancer. 2019;120(6):612–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liegl B, Kepten I, Le C, et al. Heterogeneity of kinase inhibitor resistance mechanisms in GIST. J Pathol. 2008;216(1):64–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blay JY, Serrano C, Heinrich MC, et al. Ripretinib in patients with advanced gastrointestinal stromal tumours (INVICTUS): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2020;21(7):923–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.QINLOCK™ Product Monograph. Available at https://health-products.canada.ca/dpdbdpp/dispatch-repartition.do;jsessionid=C58B3E156C26F4E7C8136E83FC5999CA. 2020.
- 12.Australian Product Information - QINLOCK™ (ripretinib). Available at https://www.tga.gov.au/product-information-0. 2020.
- 13.Smith BD, Kaufman MD, Lu WP, et al. Ripretinib (DCC-2618) Is a Switch Control Kinase Inhibitor of a Broad Spectrum of Oncogenic and Drug-Resistant KIT and PDGFRA Variants. Cancer Cell. 2019;35(5):738–751 e739. [DOI] [PubMed] [Google Scholar]
- 14.Janku F, Abdul Razak AR, Chi P, et al. Switch Control Inhibition of KIT and PDGFRA in Patients With Advanced Gastrointestinal Stromal Tumor: A Phase I Study of Ripretinib. J Clin Oncol. 2020;38(28):3294–3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zalcberg JR, Verweij J, Casali PG, et al. Outcome of patients with advanced gastrointestinal stromal tumours crossing over to a daily imatinib dose of 800 mg after progression on 400 mg. Eur J Cancer. 2005;41(12):1751–1757. [DOI] [PubMed] [Google Scholar]
- 16.National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology (NCCN guidelines) soft tissue sarcoma 2020; Version 1.2021.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.