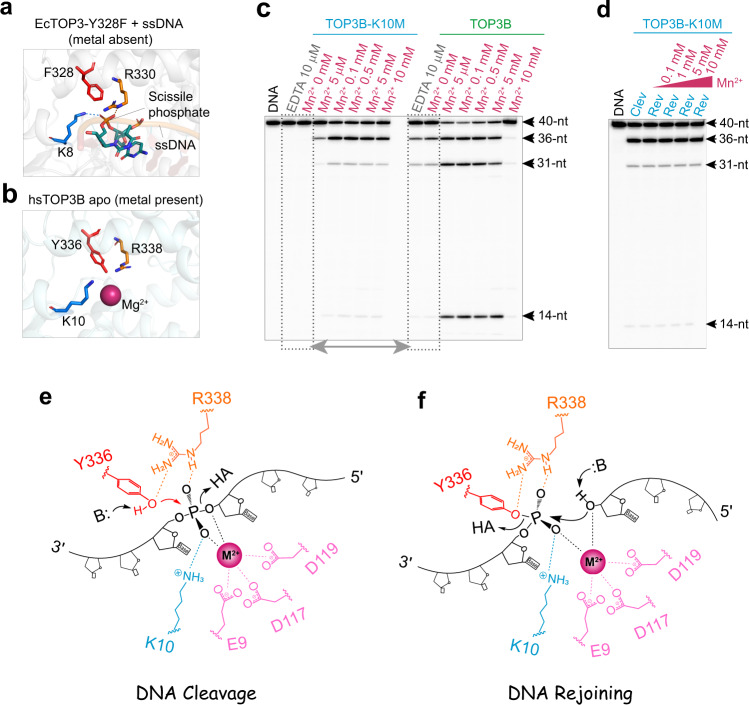
Fig. 2. TOP3B lysine 10 (K10) is a conserved catalytic residue assisting divalent cation-dependent DNA cleavage and required for DNA rejoining.
a Structure of EcTOP3 (PDB ID: 1i7D) showing the interaction between the DNA scissile phosphate and the conserved Lysine residue K8 (blue dotted line) in the absence of divalent cation. Key residues for catalysis including the Tyrosine substitute F328 and Arginine R330 are shown as sticks. b Catalytic site of hsTOP3B apo structure (PDB ID: 5gvc) in the presence of a Mg2+ ion showing the conserved Lysine K10 and other key catalytic residues. c DNA cleavage assay with WT TOP3B and mutant TOP3B-K10M without divalent cation and with the indicated concentrations of Mn2+. Dotted boxes and double-headed arrow highlight the comparison of DNA cleavage activity between the mutant and the WT enzyme without divalent-metal addition. d DNA cleavage and lack of reversal with TOP3B-K10M in the presence of Mn2+ (0.1–10 mM). DNA cleavage products were collected after 30 min incubation. Reversal assays were conducted by adding high salt and indicated amount of Mn2+ for 5 min (Clev: Cleavage; Rev: Reversal). DNA cleavage assays in (c, d) were repeated twice independently with similar results. e Proposed mechanism for DNA cleavage by TOP3B. K10 and R338 of TOP3B coordinate two oxygen atoms in the DNA scissile phosphate, aligning the DNA substrate and facilitating the binding of a divalent cation (red-violet sphere). A properly bound metal ion, together with K10 and R338, stabilizes two non-bridging oxygen atoms and the 3’-oxygen leaving group (dotted lines), facilitating the nucleophilic attack by Y336 (red arrow) and subsequent phosphoryl transfer, resulting in a Tyrosyl phosphate group at the 5’-end of the cleaved DNA. f DNA-end re-alignment and rejoining. K10 and R338 play essential roles in re-aligning the 5’ DNA end (Tyrosyl phosphate) and facilitating divalent-metal binding. The captured metal ion further aligns the 3’-OH group as a nucleophile to trigger a reverse phosphoryl transfer to reform the DNA backbone. The potential general acid (HA) and base (B:) in (e, f) can be adjacent water molecules.