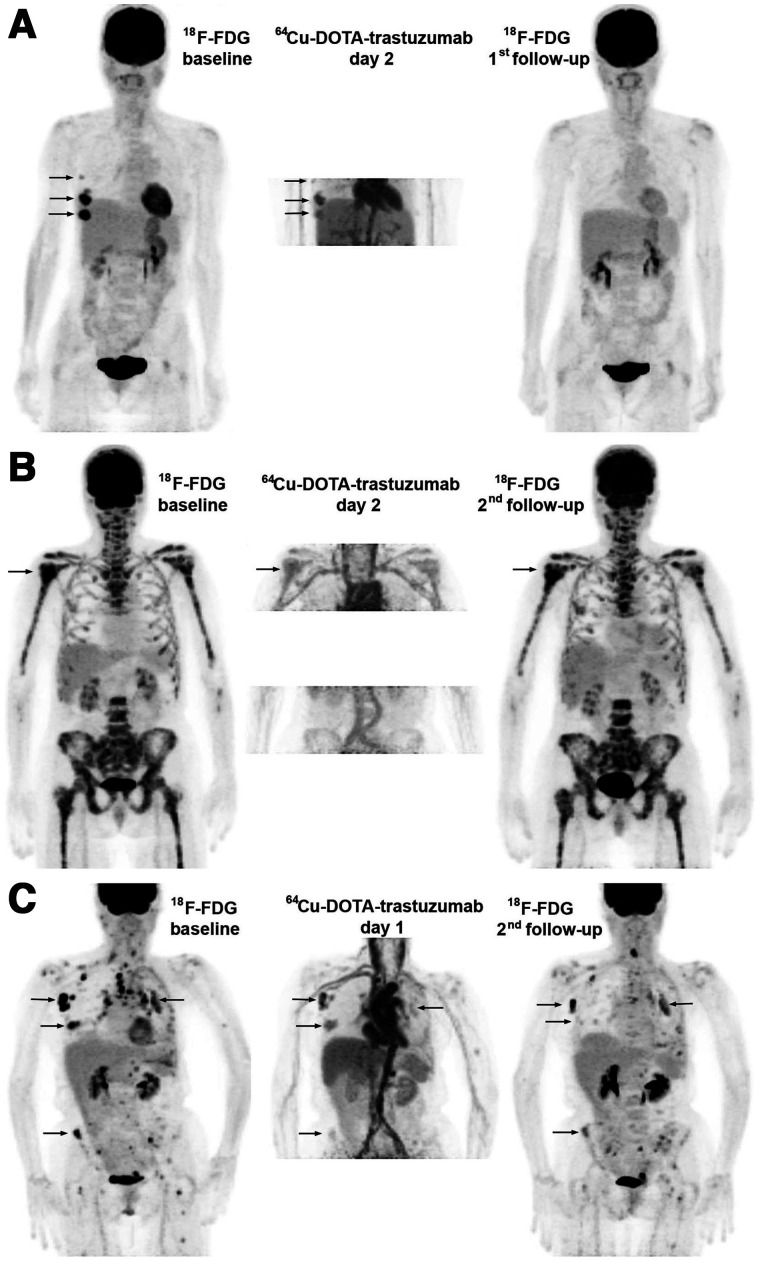
FIGURE 1.
Treatment effects. Images are maximum-intensity projections with upper intensity thresholds (black color) corresponding to SUV of 7 and 10 g/mL for 18F-FDG and 64Cu-DOTA-trastuzumab, respectively. (A) Response with baseline 18F-FDG–positive disease limited to right breast and axilla (arrows). All lesions were well visualized with 64Cu-DOTA-trastuzumab, and follow-up 18F-FDG showed complete response. (B) Nonresponse with extensive 18F-FDG–positive bone metastasis. (Arrow indicates PERCIST target tumor.) Tumor uptake of 64Cu-DOTA-trastuzumab was low (day 2 target tumor SUVmax, 5.5 g/mL). Disease progression occurred after 4 cycles of T-DM1. (C) Nonresponse with widely disseminated 18F-FDG–positive disease. Tumor uptake of 64Cu-DOTA-trastuzumab was variable, and tumor response at second 18F-FDG follow-up (after 4 cycles of T-DM1) was correspondingly mixed.