Fig. 3 |. Autoantibody access to CNS targets.
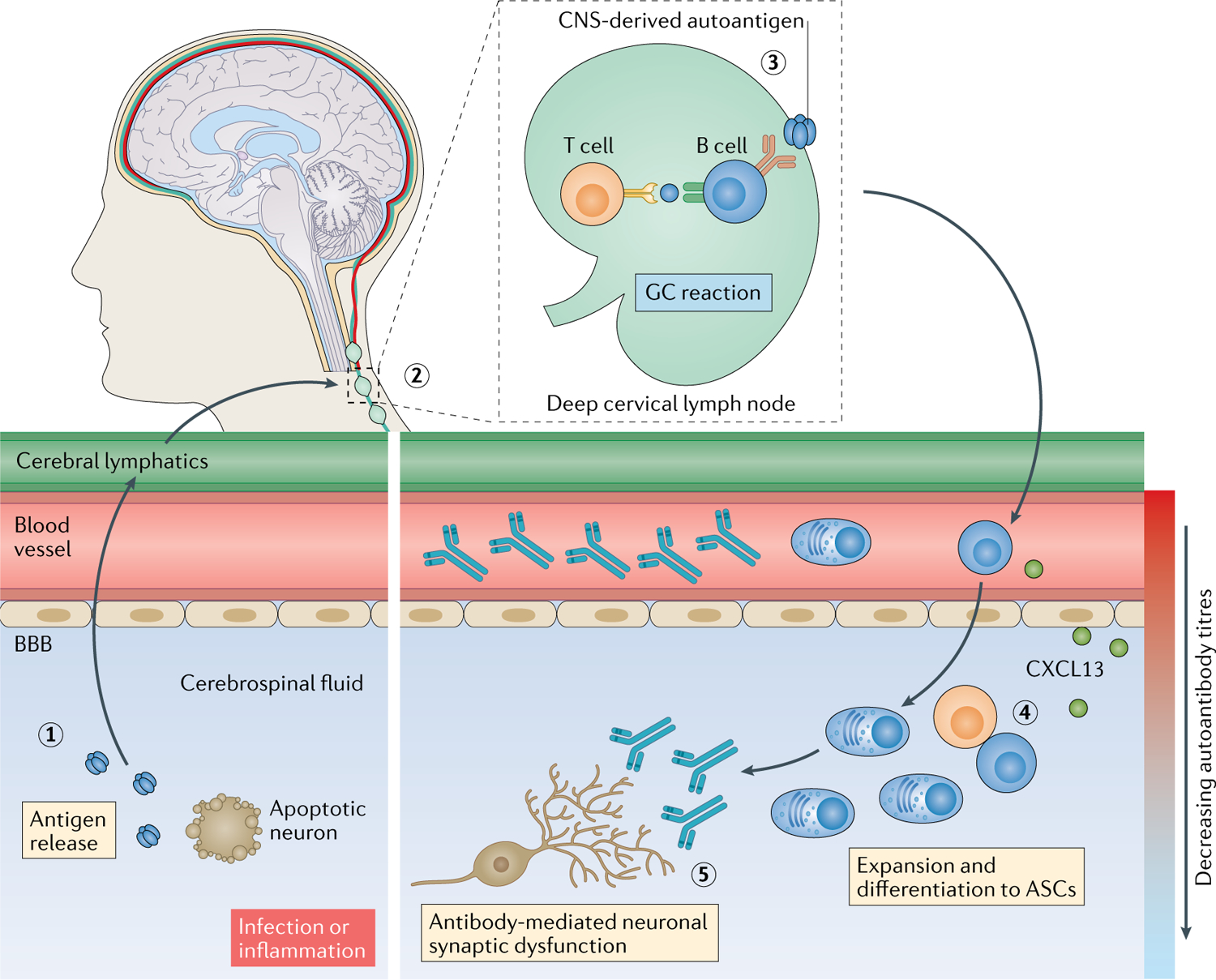
Tissue destruction, either as a result of infection112 or a pro-inflammatory milieu, can release soluble neuronal autoantigens into the cerebrospinal fluid (1), from where they can drain via the recently (re)discovered cerebral lymphatic drainage system173,174 into cervical lymph nodes (2). Here, B cell responses can be induced against CNS-derived autoantigens, generating antigen-specific B cells, plasma cells and high concentrations of autoantibodies in circulation (3). Activated B cells can then migrate into the intrathecal compartment, driven, for example, by a gradient of the C–X–C motif chemokine ligand 13 (CXCL13). In the intrathecal compartment, clonal expansion, class switching, affinity maturation and differentiation into antibody-secreting cells (ASCs) that secrete pathogenic autoantibodies can occur (4) and can be associated with ectopic tertiary lymphoid structures. These intrathecal antibodies can directly lead to neuronal dysfunction (5). BBB, blood–brain barrier; GC, germinal centre.
