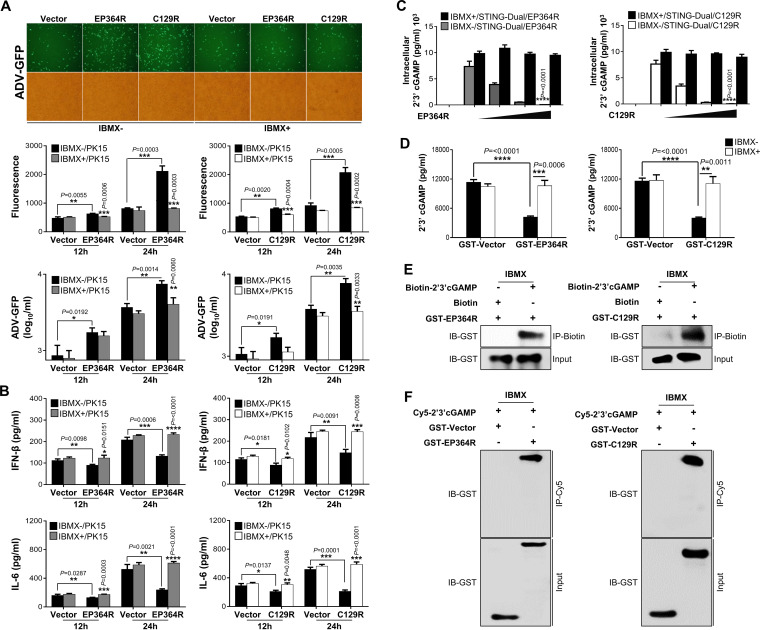
FIG 5.
Phosphodiesterase activity of EP364R and C129R inhibits activation of STING by degrading 2′,3′-cGAMP. (A and B) PK-15 cells were transfected with Flag-EP364R and Flag-C129R and treated with 200 nM IBMX at 6 hpt and infected with ADV-GFP (MOI = 1) at 24 hpt. Fluorescence microscopy and fluorescence absorbance and virus replication (A) and IFN-β secretion and IL-6 secretion (B) were measured at indicated time points. (C) 293-Dual hSTING-A162 cells were cotransfected with 3×Flag-cGAS plasmid and indicated plasmids dose dependently, and 6 h before cells were harvested, they were treated with 100 nM IBMX. Cells were harvested 24 hpt, and the intracellular 2′,3′-cGAMP level was measured. (D) In vitro 2′,3′-cGAMP degradation assay. Purified GST-EP364R or GST-C129R proteins plus 2.5 μM 2′,3′-cGAMP with or without 1 mM IBMX were incubated for 22 h at 37°C in a reaction mixture. Then 2′,3′-cGAMP level in each sample was quantified by 2′,3′-cGAMP ELISA. (E and F) GST-purified EP364R and C129R protein interaction with biotin-cGAMP or Cy5-cGAMP. (E) Here, 2 μg of ASFV proteins was incubated with 10 μM biotin-cGAMP or 10 μM biotin in a reaction buffer for 2 h at 30°C with the presence of 1 mM IBMX. Biotinylated cGAMP was pulled down by streptavidin magnetic beads followed by immunoblotting with anti-GST antibody. (F) ASFV GST-purified protein with vector was incubated with Cy5-cGAMP and subjected to Cy5 pulldown using anti-Cy5 antibody followed by immunoblotting with anti-GST antibody. All the data are representative of at least two independent experiments, each with similar results, and the values are expressed as means and SD for three biological replicates. All the immunoblot data are representative of at least two independent experiments, each with similar results. Student's t test: *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001.