Abstract
The κ-opioid receptor (KOR) is implicated in various neuropsychiatric disorders. We previously evaluated an agonist tracer, 11C-GR103545, for PET imaging of KOR in humans. Although 11C-GR103545 showed high brain uptake, good binding specificity, and selectivity for KOR, it displayed slow kinetics and relatively large test–retest variability of total distribution volume (VT) estimates (15%). Therefore, we set out to develop 2 novel KOR agonist radiotracers, 11C-EKAP and 11C-FEKAP. In nonhuman primates, both tracers exhibited faster kinetics than 11C-GR103545 and comparable binding parameters to 11C-GR103545. The aim of this study was to assess their kinetic and binding properties in humans. Methods: Six healthy subjects underwent 120-min test–retest PET scans with both 11C-EKAP and 11C-FEKAP. Metabolite-corrected arterial input functions were measured. Regional time–activity curves were generated for 14 regions of interest. One-tissue-compartment and 2-tissue-compartment (2TC) models and the multilinear analysis-1 (MA1) method were applied to the regional time–activity curves to calculate VT. The time stability of VT and test–retest reproducibility were evaluated. Levels of specific binding, as measured by the nondisplaceable binding potential (BPND) for the 3 tracers (11C-EKAP, 11C-FEKAP, and 11C-GR103545), were compared using a graphical method. Results: For both tracers, regional time–activity curves were fitted well with the 2TC model and MA1 method (t* = 20 min) but not with the 1-tissue-compartment model. Given the unreliably estimated parameters in several fits with the 2TC model and a good VT match between MA1 and 2TC, MA1 was chosen as the appropriate model for both tracers. Mean MA1 VT was highest for 11C-GR103545, followed by 11C-EKAP and then 11C-FEKAP. The minimum scan time for stable VT measurement was 90 and 110 min for 11C-EKAP and 11C-FEKAP, respectively, compared with 140 min for 11C-GR103545. The mean absolute test–retest variability in MA1 VT estimates was 7% and 18% for 11C-EKAP and 11C-FEKAP, respectively. BPND levels were similar for 11C-FEKAP and 11C-GR103545 but were about 25% lower for 11C-EKAP. Conclusion: The 2 novel KOR agonist tracers showed faster tissue kinetics than 11C-GR103545. Even with a slightly lower BPND, 11C-EKAP is judged to be a better tracer for imaging and quantification of KOR in humans, on the basis of the shorter minimum scan time and the excellent test–retest reproducibility of regional VT.
Keywords: PET, kinetic modeling, receptor imaging, brain imaging, κ-opioid receptors
The κ-opioid receptors (KOR) have been implicated in various psychiatric disorders, including addictions, depression, and related mood disorders. We have previously developed and evaluated a set of KOR agonist and antagonist tracers, 11C-GR103545 and 11C-LY2795050, for PET imaging of receptors in humans (1,2). The antagonist tracer, 11C-LY2795050, proved to be suitable for imaging and quantifying KOR in the human brain (3–5). However, the agonist radiotracer, 11C-GR103545, required a long scan time (140 min) for quantification of binding parameters due to its slow kinetics (6). In addition, the variability of outcome measures was higher than desirable (e.g., 15% variability on the total distribution volume [VT] of the test–retest study (6)). Therefore, KOR agonist tracers with faster kinetics and improved imaging properties are needed for reliable quantification of KOR configured in the high-affinity state (7). Such agonist tracers are needed to complement the antagonist tracer, which can be used to image and quantify the total KOR levels. We have developed 2 new agonist radiotracers, 11C-EKAP (8) and 11C-FEKAP (9) (Fig. 1). Evaluation in nonhuman primates showed that they indeed have faster kinetics than 11C-GR103545, with comparable binding specificity. In this paper, we report the first-in-humans evaluation of 11C-EKAP and 11C-FEKAP to establish the appropriate kinetic models for analysis of imaging data and to assess the test–retest reproducibility of binding parameters. The kinetic and binding properties of 11C-EKAP and 11C-FEKAP were also compared with those of 11C-GR103545.
FIGURE 1.
Molecular structures of C-EKAP, 11C-FEKAP, and 11C-GR103545.
MATERIALS AND METHODS
Human Subjects
Six healthy subjects (20–51 y old; 3 men and 3 women; body weight, 75 ± 13 kg) were enrolled in the study. PET imaging experiments were conducted under a protocol approved by the Yale University School of Medicine Human Investigation Committee and the Yale–New Haven Hospital Radiation Safety Committee and were in accordance with U.S. federal guidelines and regulations for the protection of human research subjects (title 45, part 46, of the Code of Federal Regulations). Written informed consent was obtained from all subjects. All were healthy, as assessed by a physical examination, a comprehensive metabolic panel, a complete blood count, and medical and psychiatric histories. The subjects had no current prescription or illicit drug use, no history of tobacco or nicotine use, no current uncontrolled medical conditions, and no history of neurologic or psychiatric disorders. Women had negative pregnancy tests at intake and on the day of the scans. MR images of all subjects were acquired for verification of no structural brain abnormalities and for PET image registration. MRI was performed on a 3-T whole-body scanner (Trio; Siemens Medical Systems) with a circularly polarized head coil. The dimension and pixel size of MR images were 256 × 256 × 176 voxels and 0.98 × 0.98 × 1.0 mm3, respectively.
Radiotracer Synthesis
11C-EKAP and 11C-FEKAP were synthesized as previously described (8,9). The radiochemical purity of 11C-EKAP and 11C-FEKAP in the final product solution was more than 98%.
PET Imaging Experiments
Each subject underwent 2 PET scans: one with 11C-EKAP and the other with 11C-FEKAP. On the test day, the subjects underwent 11C-EKAP PET first and 11C-FEKAP PET second. On the retest day, the subjects underwent 11C-FEKAP first and 11C-EKAP second, except for 1 subject who underwent the retest scans a week apart. The test and retest scans were 11 ± 11 d apart for 11C-EKAP and 10 ± 8 d apart for 11C-FEKAP, except for 1 subject, who completed the retest scan 69 d after the test scan. All PET scans were conducted on a High Resolution Research Tomograph (Siemens Medical Solutions), which acquires 207 slices (1.2-mm slice separation) with a reconstructed image resolution of about 3 mm. After a 6-min transmission scan for attenuation correction, PET scans were acquired for 120 min in list mode after intravenous administration of 11C-EKAP or 11C-FEKAP over 1 min by an automatic pump (PHD 22/2000; Harvard Apparatus). The injected mass limit was 0.02 μg/kg of body weight. Dynamic scan data were reconstructed into 33 frames (6 × 0.5 min, 3 × 1 min, 2 × 2 min, and 22 × 5 min) with corrections for attenuation, normalization, scatter, randoms, and dead time using the MOLAR algorithm (a motion-compensation ordered-subsets expectation maximization list-mode algorithm for resolution-recovery reconstruction (10)). The reconstruction included event-by-event motion correction (11) based on measurements with the Polaris Vicra sensor (NDI Systems), with reflectors being mounted on a swim cap worn by the subject.
Input Function Measurement
The radial artery was catheterized for blood sampling. Manual 0.5-mL samples were collected every 10 s for the first 90 s; thereafter, 21 samples (0.5–10 mL) were collected manually at selected time points. Plasma was obtained by centrifugation at 4°C (2,930g for 5 min). Whole blood and plasma were counted in cross-calibrated γ-counters (Wizard 1480 and 2480; PerkinElmer). To reduce noise in the data, the total plasma curve from approximately 5 min onward was fitted to a sum of exponentials.
Plasma Metabolite Analysis
Radioactive metabolites in the arterial plasma were analyzed using a modified automatic column-switching high-performance liquid chromatography method (12). Plasma samples collected at 5, 15, 30, 60, and 90 min after injection were mixed with urea (8 M) and then filtered through 1.0-μm Whatman 13-mm GD/X syringe filters (GE Healthcare). Up to 5 mL of plasma filtrate were injected onto the automatic column-switching high-performance liquid chromatography system connected to a capture column (4.6 × 19 mm) self-packed with Phenomenex Strata-X polymeric solid-phase extraction sorbent and eluting with 1% MeCN in water at 2 mL/min for 4 min. The trapped activity in the capture column was then back flushed and eluted through a Phenomenex Luna C18 phenyl hexyl analytic column (4.6 × 250 mm, 5 μm) with a mobile phase consisting of 45% MeCN and 55% 0.1 M ammonium formate (v/v) at a flow rate of 1.8 mL/min. High-performance liquid chromatography eluate was fraction-collected and counted in the γ-counters. The fraction counts were corrected for volume and decay. The unmetabolized parent fraction was calculated as the ratio of the sum of radioactivity in fractions containing the parent compound to the total amount of radioactivity collected and was fitted to an integrated γ-function (4 fitted parameters: a, b, c, and d):
In addition, the time-varying extraction efficiency of radioactivity in filtered plasma samples was determined and normalized to that of reference plasma sample. The plasma input function was obtained as the product of the total plasma activity, the parent fraction, and the normalized extraction efficiency.
Measurement of Tracer Free Fraction in Plasma
Arterial blood samples were taken immediately before tracer injection for analysis of the plasma free fraction (fP). An ultrafiltration method (Centrifree micropartition device 4104A; Millipore) was used for measuring the fP of tracer in plasma in triplicate. The fP was determined from the count ratio of ultrafiltrate to plasma.
Image Registration and Definition of Regions of Interest
Regions of interest were defined in the automated anatomic labeling for SPM2 (13) in Montreal Neurologic Institute space (14). After hardware motion correction, the dynamic PET images were coregistered to the early summed PET images from 0 to 10 min after injection using a 6-parameter mutual information algorithm (15) (FMRIB’s Linear Image Registration Tool; Functional Magnetic Resonance Imaging of the Brain [FMRIB] Software Library) to eliminate any residual motion. The summed PET image was then coregistered to the individual subject’s T1-weighted 3-T MR image (6-parameter rigid registration) and then coregistered to the automated anatomic labeling template in Montreal Neurologic Institute space using a nonlinear transformation (Bioimage suite) (16). Using the combined transformations from the template to the individual subject’s PET space, regional time–activity curves were generated for 14 regions of interest: amygdala, caudate, centrum semiovale, cerebellum, anterior cingulate cortex, frontal cortex, globus pallidus, hippocampus, insula, occipital cortex, posterior cingulate cortex, putamen, temporal cortex, and thalamus.
Quantitative Analysis
The outcome measures were derived with kinetic analysis of the regional time–activity curves using the arterial plasma input function. VT (17) was calculated using 1- and 2-tissue compartment (1TC and 2TC, respectively) models and the multilinear analysis-1 (MA1) method (18). The test scans (n = 6) were used for kinetic model assessment. The time stability of VT estimates was assessed by comparing VT from shortened scans (ranging from 110 to 50 min) to the 120-min VT using the MA1 (t* = 20 min) model. The ratio of VT from the shortened scan to that from the 120-min scan was computed for each region of interest and duration. Two criteria were adopted to determine a minimum scan duration (19): The first criterion was that the average of the ratio was between 0.95 and 1.05. The second criterion was that the interindividual SD of the ratio was less than 0.1. All modeling was performed with in-house programs written with Interactive Data Language (version 8.0; ITT Visual Information Solutions). For parameter estimation, data points were weighted on the basis of the noise-equivalent counts in each frame. Percentage SE (%SE) was estimated from the theoretic parameter covariance matrix. %SE was used to examine the reliability of individual fits (fits were considered unreliable when %SE of VT was >10%).
Because KOR is distributed throughout the brain, no reference region was available. To predict which KOR radiotracer will show higher specific binding signals, the graphical method of Guo et al. (Guo plot) (20) was applied to compare 11C-EKAP and 11C-FEKAP with 11C-GR103545. The equation for the Guo plot to compare tracer A and tracer B is…
When plotting VTB (y-axis) against VTA (x-axis), the sign of the y-intercept predicts which tracer will produce a bigger BPND. The mean VT across test scans for 11C-EKAP and 11C-FEKAP and the mean VT from a previous study (6) for 11C-GR103545 were used for the Guo plot. The regression line was estimated with the total least-squares method using weights that are proportional to the inverse of intersubject SD. The relative BPND can be estimated from the measured intercept if the VND of tracer B is known.
For the test–retest data, results were evaluated according to 3 criteria: relative test–retest variability (TRV), absolute TRV (aTRV), and intraclass correlation coefficient (ICC). The test and retest scans that were more than 1 mo apart (n = 1) were excluded. TRV was calculated as the difference between the parameters in the test and retest scans divided by their average. The mean of TRV denotes the presence of a trend between the 2 scans, and the SD of TRV is an index of the variability in the difference of 2 estimates. aTRV is the absolute value of TRV and comparable to the error in a single measurement.
RESULTS
Injection Parameters and Plasma Analysis
The mean (±SD) of the administered mass of 11C-EKAP and 11C-FEKAP was 1.18 ± 0.32 μg (range, 0.80–1.65 μg) and 1.12 ± 0.35 μg (range, 0.62–1.61 μg), respectively. The mean administered activity of 11C-EKAP and 11C-FEKAP was 580 ± 111 MBq (range, 382–746 MBq) and 483 ± 197 MBq (range, 152–730 MBq), respectively. There were no adverse or clinically detectable pharmacologic effects in any of the 6 subjects. No significant changes in vital signs or the results of laboratory studies were observed.
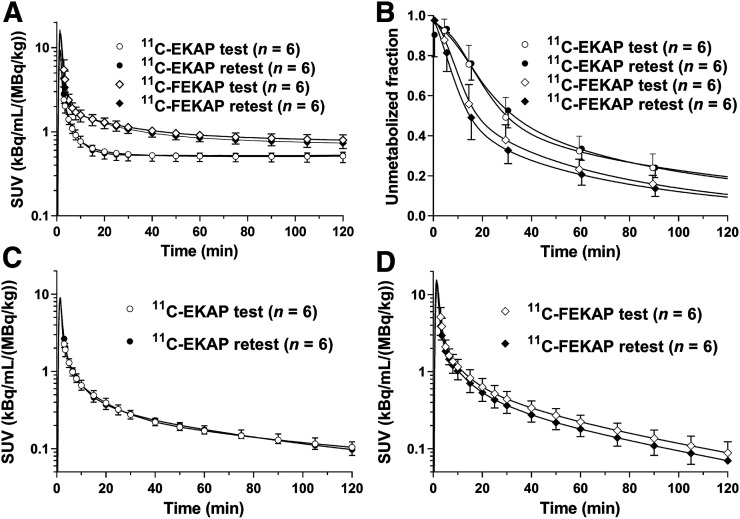
Table 1 lists the injected radioactivity dose, molar activity at the time of injection, injected mass, and fP. There were no significant differences in injected dose, injected mass, or fP between the test and retest scans with either tracer. Figure 2 displays the parent fractions and metabolite-corrected plasma curves from the test–retest study for both tracers. The parent fractions were similar between the test and retest scans for both tracers. 11C-FEKAP displayed a lower parent fraction than 11C-EKAP in plasma. The mean parent fractions at 60 min after injection were 32% ± 7% and 34% ± 6% for the test and retest scans with 11C-EKAP and 23% ± 5% and 21% ± 5% for the test and retest scans with 11C-FEKAP. However, the actual parent radioactivity levels of the 2 tracers were quite similar (Figs. 2C and D), suggesting that the difference in parent fraction was due to different clearance rates for the radiolabeled metabolites. The fP was 0.25% ± 0.03% for 11C-EKAP (n = 12) and 0.06% ± 0.01% for 11C-FEKAP (n = 12).
TABLE 1.
Subject Information and PET Scan Parameters
|
11C-EKAP |
11C-FEKAP |
|||||
| Parameter | Test | Retest | P | Test | Retest | P |
| Injected dose (MBq) | 625 ± 85 | 534 ± 121 | 0.07 | 447 ± 180 | 519 ± 223 | 0.38 |
| Molar activity at time of injection (MBq/nmol) | 244 ± 63 | 205 ± 84 | 0.35 | 188 ± 119 | 237 ± 126 | 0.43 |
| Injected mass (μg)* | 1.15 ± 0.31 | 1.20 ± 0.35 | 0.71 | 1.21 ± 0.39 | 1.03 ± 0.32 | 0.34 |
| f P | 24.6% ± 2.8% | 24.5% ± 3.1% | 0.96 | 6.3% ± 0.9% | 6.2% ± 0.8% | 0.73 |
Mass limit, 0.02 μg/kg.
n = 6/group.
FIGURE 2.
Mean ± SD of total plasma activity (A), parent fraction in plasma (B), metabolite-corrected plasma activity over time after injection of 11C-EKAP (C), and metabolite-corrected plasma activity over time after injection of 11C-FEKAP (D). SUV is [concentration/(injected dose/body weight)].
Modeling Results
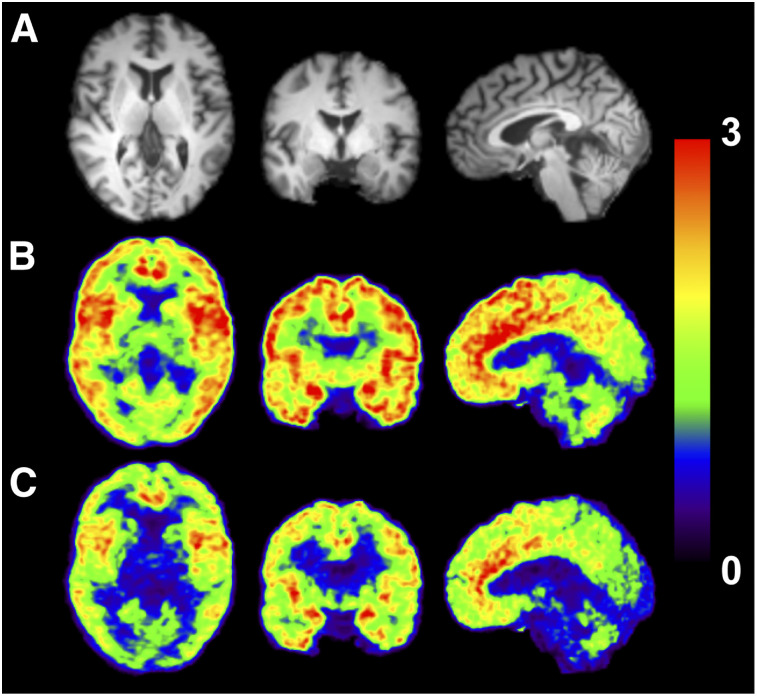
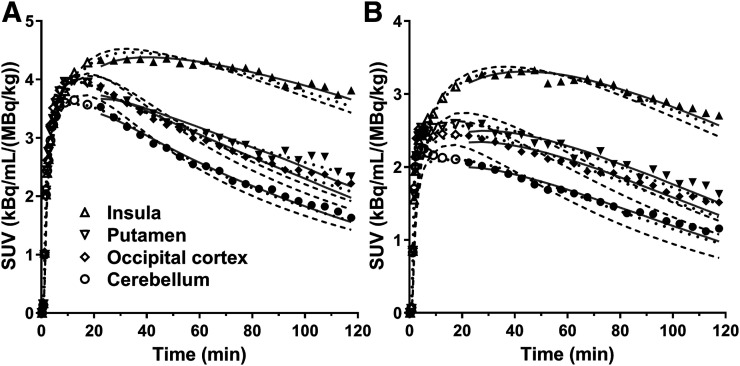
Radioactivity distribution in the brain was heterogeneous, and the distribution pattern was similar between 11C-EKAP and 11C-FEKAP (Fig. 3). Regional time–activity curves for representative brain regions are shown in Figure 3. The time–activity curves peaked at about 20 and 40 min after injection of 11C-EKAP and 11C-FEKAP, respectively (Fig. 4). Typical examples of curve fittings with 1TC and 2TC models and MA1 are also shown in Figure 4. Regional time–activity curves were fitted well with the 2TC and MA1 models, and to a lesser extent with the 1TC model. The parameters of the 2TC model were not reliably estimated (%SE > 10% in VT) in a few cases, especially in the amygdala. Given the low ICC of MA1 VT for 11C-EKAP in the amygdala, quantification was still difficult in the amygdala even with MA1, because of the combination of the small region-of-interest size and slow kinetics. Mean K1 (mL/cm3/min) in the 1TC model ranged from 0.09 (centrum semiovale) to 0.21 (putamen) for 11C-EKAP and from 0.033 (centrum semiovale) to 0.076 (insula) for 11C-FEKAP. There were excellent correlations in VT between the kinetic models (11C-EKAP: VT(1TC) = 0.96 × VT(2TC) − 0.53, R2 = 0.98, and VT(MA1, t* = 20 min) = 1.02 × VT(2TC) − 0.28, R2 = 0.98; 11C-FEKAP: VT(1TC) = 0.92 × VT(2TC) − 0.17, R2 = 0.98, and VT(MA1, t* = 20 min) = 1.02 × VT(2TC) + 0.11, R2 = 1.00). These comparisons were performed for the regions with good identifiability, that is, %SE of VT < 10% with the 2TC model. For both tracers, t* for MA1 was selected as 20 min by comparing the MA1 VT with 2TC VT. On the basis of the good and consistent quality of fit and comparison with 2TC VT, the MA1 model was chosen for both tracers.
FIGURE 3.
Images from typical subject (male, 51 y old, 82-kg body weight). (A) MR images. (B and C) Coregistered PET images summed from 30 to 90 min after injection of 11C-EKAP (B) or 11C-FEKAP (C). SUV is [concentration/(injected dose/body weight)].
FIGURE 4.
Regional time–activity curves in 4 regions of interest after injection of 11C-EKAP (A) and 11C-FEKAP (B) with 1TC (dashed), 2TC (dotted), and MA1 (solid) fits. For each region, symbols correspond to measured regional activity.
Regional VT estimated using 1TC, 2TC, and MA1 (t* = 20 min) and the minimum scan time for the MA1 model is summarized in Table 2. For both 11C-EKAP and 11C-FEKAP, a high VT was seen in the amygdala, insula, and anterior cingulate cortex, and a lower VT was seen in the centrum semiovale, cerebellum, and thalamus. The intersubject VT variability was higher for 11C-FEKAP (MA1, 23%–39%) than 11C-EKAP (MA1, 14%–26%). The minimum scan durations to obtain a stable VT were 90 and 110 min for 11C-EKAP and 11C-FEKAP, respectively.
TABLE 2.
Regional VT in Test Scans
| Regional VT (mL/cm3) |
Minimum scan duration (min) |
|||||||
|
11C-EKAP (n = 6) |
11C-FEKAP (n = 6) |
11C-EKAP (n = 6) | 11C-FEKAP (n = 6) | |||||
| Region | 1TC | 2TC | MA1 | 1TC | 2TC | MA1 | MA1 | MA1 |
| Amygdala | 19.9 (22%) | 18.1* (6%) | 21.6 (25%) | 9.1 (33%) | 9.0† (39%) | 9.6 (36%) | 90 | 90 |
| Insula | 13.9 (21%) | 14.7 (19%) | 15.2 (21%) | 6.4 (27%) | 6.8‡ (31%) | 7.1 (28%) | 80 | 70 |
| Anterior cingulate cortex | 13.1 (13%) | 13.8 (13%) | 14.2 (14%) | 5.8 (21%) | 6.2 (22%) | 6.5 (23%) | 70 | 70 |
| Globus pallidus | 10.7 (19%) | 11.5 (18%) | 11.7 (19%) | 4.6 (28%) | 5.3 (29%) | 5.4 (30%) | 70 | 60 |
| Temporal cortex | 9.8 (18%) | 11.0 (21%) | 10.7 (18%) | 4.3 (25%) | 4.8 (27%) | 5.0 (28%) | 80 | 90 |
| Putamen | 9.3 (18%) | 10.8 (21%) | 10.3 (18%) | 3.9 (23%) | 4.6 (26%) | 4.8 (26%) | 80 | 100 |
| Frontal cortex | 9.0 (19%) | 10.3 (23%) | 9.9 (19%) | 4.1 (25%) | 4.6 (27%) | 4.8 (27%) | 80 | 90 |
| Hippocampus | 7.6 (21%) | 9.1 (26%) | 8.8 (22%) | 3.2 (23%) | 3.8 (27%) | 4.1 (27%) | 80 | 90 |
| Occipital cortex | 7.5 (20%) | 8.7 (21%) | 8.3 (19%) | 3.2 (26%) | 3.8 (29%) | 3.9 (30%) | 80 | 90 |
| Caudate | 7.5 (24%) | 8.1 (26%) | 8.0 (24%) | 2.9 (27%) | 3.4 (29%) | 3.5 (30%) | 50 | 80 |
| Posterior cingulate cortex | 6.8 (27%) | 7.2‡ (25%) | 7.6 (26%) | 2.7 (29%) | 3.1 (30%) | 3.3 (30%) | 80 | 100 |
| Cerebellum | 5.7 (32%) | 6.6 (29%) | 6.4 (29%) | 2.1 (34%) | 2.6‡ (32%) | 3.0 (37%) | 80 | 100 |
| Thalamus | 4.8 (20%) | 5.2‡ (15%) | 5.4 (20%) | 1.5 (22%) | 1.9‡ (20%) | 2.3 (39%) | 80 | 110 |
n = 4 (relative SE > 10% was excluded).
n = 2 (relative SE > 10% was excluded).
n = 5 (relative SE > 10% was excluded).
Data in parentheses are percentage coefficient of variation across subjects.
Test–Retest Reproducibility
The aTRV of MA1 VT estimates was good (4%–8%) for 11C-EKAP across all regions except the amygdala (17%) (Table 3). The aTRV of 11C-FEKAP VT was higher in all regions (13%–26%) than was the aTRV of 11C-EKAP. The test–retest reproducibility of VT measured by ICC was excellent (0.78–0.98) for 11C-EKAP in all regions except the amygdala (0.19). The ICC of 11C-FEKAP was also generally good (0.63–0.83) except in 3 regions: anterior cingulate cortex (0.57), cerebellum (0.47), and thalamus (0.55).
TABLE 3.
TRV and Reproducibility of VT
|
11C-EKAP |
11C-FEKAP |
|||||
| Region | aTRV (%) | TRV (%) | ICC | aTRV (%) | TRV (%) | ICC |
| Amygdala | 17 ± 14 | 4 ± 23 | 0.19 | 24 ± 11 | −12 ± 26 | 0.72 |
| Insula | 7 ± 6 | 1 ± 10 | 0.85 | 19 ± 9 | −5 ± 23 | 0.63 |
| Anterior cingulate cortex | 7 ± 5 | 3 ± 9 | 0.78 | 17 ± 6 | −2 ± 20 | 0.57 |
| Globus pallidus | 8 ± 4 | 2 ± 10 | 0.82 | 16 ± 5 | −4 ± 18 | 0.83 |
| Temporal cortex | 6 ± 5 | 0 ± 8 | 0.91 | 17 ± 5 | −1 ± 20 | 0.68 |
| Putamen | 6 ± 6 | −3 ± 9 | 0.86 | 13 ± 5 | −2 ± 16 | 0.79 |
| Frontal cortex | 5 ± 6 | −1 ± 8 | 0.91 | 16 ± 5 | −3 ± 19 | 0.71 |
| Hippocampus | 5 ± 5 | −1 ± 8 | 0.92 | 17 ± 7 | −5 ± 19 | 0.72 |
| Occipital cortex | 4 ± 5 | −1 ± 6 | 0.96 | 17 ± 5 | −2 ± 19 | 0.73 |
| Caudate | 6 ± 7 | −3 ± 9 | 0.95 | 17 ± 5 | −3 ± 19 | 0.78 |
| Posterior cingulate cortex | 7 ± 6 | −1 ± 10 | 0.95 | 18 ± 8 | −3 ± 21 | 0.74 |
| Cerebellum | 6 ± 5 | 0 ± 8 | 0.98 | 26 ± 14 | 0 ± 32 | 0.47 |
| Thalamus | 5 ± 7 | −2 ± 8 | 0.87 | 20 ± 14 | 3 ± 26 | 0.55 |
n = 5/group.
Comparison of 11C-EKAP and 11C-FEKAP with 11C-GR103545
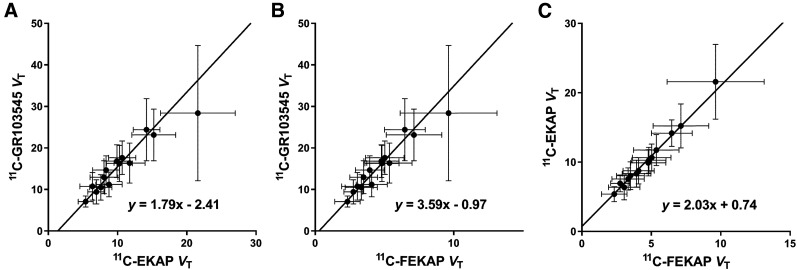
Figure 5 shows the Guo plots to compare the regional VT of 11C-EKAP, 11C-FEKAP, and 11C-GR103545. An excellent linear relationship was observed among VT values across regions, suggesting that the tracers bind to the same target with the same distribution. On the basis of the y-intercepts in Figure 5, 11C-GR103545 has the highest binding potential, followed by 11C-FEKAP and then 11C-EKAP. The regression yielded a negative y-intercept versus 11C-GR103545 (11C-EKAP, −2.41;11C-FEKAP, −0.97). Using the mean population nondisplaceable VT of 11C-GR103545 (VND, 3.4 mL/cm3) and a BPND range (1.1–7.4) taken from the literature for 11C-GR103545, regional BPND was estimated to range from 0.6 to 4.3 for 11C-EKAP and from 0.8 to 5.7 for 11C-FEKAP. The ratio of BPND (11C-EKAP) to BPND (11C-FEKAP) was 0.75.
FIGURE 5.
Comparisons of VT for 11C-EKAP, 11C-FEKAP, and 11C-GR103545. Error bars show intersubject variability (SD).
DISCUSSION
We evaluated the kinetics of 2 novel KOR agonists, 11C-EKAP and 11C-FEKAP, as PET radiotracers in humans, in comparison with 11C-GR103545, an agonist tracer previously reported by us (6).
Three kinetic models for 11C-EKAP and 11C-FEKAP were compared with arterial input functions. Regional time–activity curves were fitted well by the 2TC model and MA1 for both tracers. VT was nearly identical between the 2TC model and MA1. As seen for 11C-GR103545 (6), the 2TC model produced VT estimates with large errors in some fits, especially in the amygdala. On the other hand, the MA1 method estimated VT reliably in all fits and produced similar VT values for a t* setting of 10–30 min. For both tracers, MA1 is he model of choice. For the same reason, MA1 (t* = 40 min) was also selected for 11C-GR103545.
The rank order of VT and tracer uptake pattern was the same between 11C-EKAP, 11C-FEKAP, and 11C-GR103545. As seen with 11C-GR103545, the thalamus had the lowest VT for both 11C-EKAP and 11C-FEKAP. For the 1TC K1 (mL/cm3/min), 11C-EKAP showed the highest K1 (0.09–0.21), followed by 11C-GR103545 (0.06–0.14) and 11C-FEKAP (0.033–0.076). For the MA1 VT (mL/cm3), 11C-GR103545 (7.3–26.9) gave the highest VT, followed by 11C-EKAP (5.4–21.6) and 11C-FEKAP (2.3–9.6).
The average TRV (aTRV) of VT was 7% (range, 4%–17%) for 11C-EKAP, 18% (range, 13%–26%) for 11C-FEKAP, and 15% (range, 8%–41%) for 11C-GR103545. The minimum scan time required for stable VT estimates was 90, 110, and 140 min for 11C-EKAP, 11C-FEKAP, and 11C-GR103545, respectively. Since these tracers are 11C-labeled, a short scan time (e.g., ≤90 min) is preferred. 11C-FEKAP, with a TRV of more than 15% in most regions, may not be useful for evaluating group differences in receptor availability.
Even though 11C-EKAP showed better reproducibility than the other 2 tracers, the fact that there were a small number of subjects (n = 5) requires a careful interpretation of the test–retest results and numeric values of TRV, aTRV, and ICC. TRV (aTRV = TRV for n = 1) for the excluded subject (whose scans were 69 d apart) was 33% ± 3% for 11C-EKAP and 16% ± 5% for 11C-FEKAP (across regions). This finding indicates that long-term variability in κ-expression might be larger than the TRV reported here. However, more data are required to verify this result.
Since KOR is ubiquitously distributed throughout the brain, there are no appropriate reference regions in humans for use in kinetic modeling, as has been demonstrated in the studies of other KOR agonist and antagonist radiotracers (3,6). Therefore, we used the Guo plot to compare the magnitude of nondisplaceable binding potential (BPND) between the 2 new agonist radiotracers. The linearity of the Guo plot indicates whether the tracers bind with the same target. An almost perfect linear relation was observed between 11C-EKAP and 11C-FEKAP VT (Fig. 5C), but the linearity between 11C-EKAP or 11C-FEKAP and 11C-GR103545 was not as good (Figs. 5A and 5B). This finding does not necessarily mean that 11C-GR103545 binds to a target different from that of the 2 new tracers, as the results for 11C-EKAP and 11C-FEKAP are from the same group of subjects who received both tracers (whereas those for 11C-GR103545 are not). As there are several regions (e.g., amygdala) with high intrasubject VT variability, weighting is required to compute the regression line of the Guo plot. We used the inverse of the intersubject SD of VT as weighting, and we used the total least-squares method to take the intersubject SD of VT for both tracers into account. The y-intercept of the Guo plot consists of VND and a ratio of BPND between the 2 tracers. By substituting the population VND (3.4 mL/cm3) and a range of BPND (1.1–7.4) from 11C-GR103545 into the y-intercept, the BPND of 11C-EKAP and 11C-FEKAP can be calculated: the relative binding potentials are 1.71 (BPND [11C-GR103545]/BPND [11C-EKAP]) and 1.28 (BPND [11C-GR103545]/BPND [11C-FEKAP]). 11C-FEKAP BPND is similar to 11C-GR103545 BPND, whereas 11C-EKAP BPND is lower. Thus, wthe specific binding of 11C-EKAP is predicted to be about 25% lower than that of 11C-FEKAP. The in vivo affinity ratio can be derived from the slope of the Guo plot and the fP (KD [11C-EKAP]/KD [11C-FEKAP] ≈ 2). The order of in vitro affinities is inverted (KD [11C-EKAP]/KD [11C-FEKAP] = 0.7 (8,9)). However, for both in vitro and in vivo studies, a KD ratio of 2 is unlikely to be significantly different from identity, and it is not uncommon that disparities in in vivo and in vitro affinity measurements are found, because of multiple factors such as measurement temperature, cell or receptor types, and experimental procedures used in vitro.
CONCLUSION
The 2 novel KOR agonist radiotracers 11C-EKAP and 11C-FEKAP display faster kinetic properties than 11C-GR103545. 11C-EKAP displays much better test–retest reproducibility and requires a shorter scan time to obtain stable VT estimates. Although 11C-EKAP is predicted to have an approximately 25% lower BPND than 11C-FEKAP, the range of BPND for 11C-EKAP is very useful (∼1–4). Therefore, 11C-EKAP is judged to be a better tracer than 11C-FEKAP for the imaging and quantification of KOR agonist binding in humans.
DISCLOSURE
This study was supported by research grants from the National Institutes of Health (R21MH092664 and R33MH092664). This publication was also made possible by CTSA grant UL1 TR000142 from the National Center for Advancing Translational Sciences (NCATS), a component of the National Institutes of Health (NIH). The contents of this article are solely the responsibility of the authors and do not necessarily represent the official view of NIH. No other potential conflict of interest relevant to this article was reported.
KEY POINTS
QUESTION: Which agonist radiotracer shows suitable kinetic properties to quantify KOR in the human brain, 11C-EKAP or 11C-FEKAP?
PERTINENT FINDINGS: The 2 novel KOR agonist tracers show faster tissue kinetics than the current tracer, 11C-GR103545. 11C-EKAP is deemed to be a better tracer for imaging and quantification of KOR, based on the shorter minimum scan time and excellent test–retest reproducibility.
IMPLICATIONS FOR PATIENT CARE:11C-EKAP shortens the scan time from 140 to 90 min.
Acknowledgments
We appreciate the excellent technical assistance of the staff at the Yale University PET Center.
REFERENCES
- 1.Zheng MQ, Nabulsi N, Kim SJ, et al. Synthesis and evaluation of 11C-LY2795050 as a kappa-opioid receptor antagonist radiotracer for PET imaging. J Nucl Med. 2013;54:455–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ravert HT, Mathews WB, Musachio JL, Scheffel U, Finley P, Dannals RF. [11C]-methyl 4-[(3,4-dichlorophenyl)acetyl]-3-[(1-pyrrolidinyl)-methyl]-1- piperazinecarboxylate ([11C]GR89696): synthesis and in vivo binding to kappa opiate receptors. Nucl Med Biol. 1999;26:737–741. [DOI] [PubMed] [Google Scholar]
- 3.Naganawa M, Zheng MQ, Nabulsi N, et al. Kinetic modeling of 11C-LY2795050, a novel antagonist radiotracer for PET imaging of the kappa opioid receptor in humans. J Cereb Blood Flow Metab. 2014;34:1818–1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Naganawa M, Zheng MQ, Henry S, et al. Test-retest reproducibility of binding parameters in humans with 11C-LY2795050, an antagonist PET radiotracer for the kappa opioid receptor. J Nucl Med. 2015;56:243–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Naganawa M, Dickinson GL, Zheng MQ, et al. Receptor occupancy of the kappa-opioid antagonist LY2456302 measured with positron emission tomography and the novel radiotracer 11C-LY2795050. J Pharmacol Exp Ther. 2016;356:260–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Naganawa M, Jacobsen LK, Zheng MQ, et al. Evaluation of the agonist PET radioligand [11C]GR103545 to image kappa opioid receptor in humans: kinetic model selection, test-retest reproducibility and receptor occupancy by the antagonist pf-04455242. Neuroimage. 2014;99:69–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shalgunov V, van Waarde A, Booij J, Michel MC, Dierckx R, Elsinga PH. Hunting for the high-affinity state of G-protein-coupled receptors with agonist tracers: theoretical and practical considerations for positron emission tomography imaging. Med Res Rev. 2019;39:1014–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li S, Zheng MQ, Naganawa M, et al. Development and in vivo evaluation of a kappa-opioid receptor agonist as a PET radiotracer with superior imaging characteristics. J Nucl Med. 2019;60:1023–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li S, Zheng MQ, Naganawa M, et al. Novel kappa opioid receptor agonist as improved PET radiotracer: development and in vivo evaluation. Mol Pharm. 2019;16:1523–1531. [DOI] [PubMed] [Google Scholar]
- 10.Carson RE, Barker WC, Liow JS, Johnson CA. Design of a motion-compensation OSEM list-mode algorithm for resolution-recovery reconstruction for the HRRT. IEEE Nucl Sci Symp Conf Rec. 2003;5:3281–3285. [Google Scholar]
- 11.Jin X, Mulnix T, Gallezot JD, Carson RE. Evaluation of motion correction methods in human brain PET imaging: a simulation study based on human motion data. Med Phys. 2013;40:102503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hilton J, Yokoi F, Dannals RF, Ravert HT, Szabo Z, Wong DF. Column-switching HPLC for the analysis of plasma in PET imaging studies. Nucl Med Biol. 2000;27:627–630. [DOI] [PubMed] [Google Scholar]
- 13.Tzourio-Mazoyer N, Landeau B, Papathanassiou D, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–289. [DOI] [PubMed] [Google Scholar]
- 14.Holmes CJ, Hoge R, Collins L, Woods R, Toga AW, Evans AC. Enhancement of MR images using registration for signal averaging. J Comput Assist Tomogr. 1998;22:324–333. [DOI] [PubMed] [Google Scholar]
- 15.Viola P, Wells WM. Alignment by maximization of mutual information. Int J Comput Vis. 1997;24:137–154. [Google Scholar]
- 16.Papademetris X, Jackowski M, Rajeevan N, Constable RT, Staib LH. Bioimage suite: an integrated medical image analysis suite. Insight J. 2006;2006:209. [PMC free article] [PubMed] [Google Scholar]
- 17.Innis RB, Cunningham VJ, Delforge J, et al. Consensus nomenclature for in vivo imaging of reversibly binding radioligands. J Cereb Blood Flow Metab. 2007;27:1533–1539. [DOI] [PubMed] [Google Scholar]
- 18.Ichise M, Toyama H, Innis RB, Carson RE. Strategies to improve neuroreceptor parameter estimation by linear regression analysis. J Cereb Blood Flow Metab. 2002;22:1271–1281. [DOI] [PubMed] [Google Scholar]
- 19.Frankle WG, Huang Y, Hwang DR, et al. Comparative evaluation of serotonin transporter radioligands 11C-DASB and 11C-McN 5652 in healthy humans. J Nucl Med. 2004;45:682–694. [PubMed] [Google Scholar]
- 20.Guo Q, Owen DR, Rabiner EA, Turkheimer FE, Gunn RN. A graphical method to compare the in vivo binding potential of PET radioligands in the absence of a reference region: application to [11C]PBR28 and [18F]PBR111 for TSPO imaging. J Cereb Blood Flow Metab. 2014;34:1162–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]