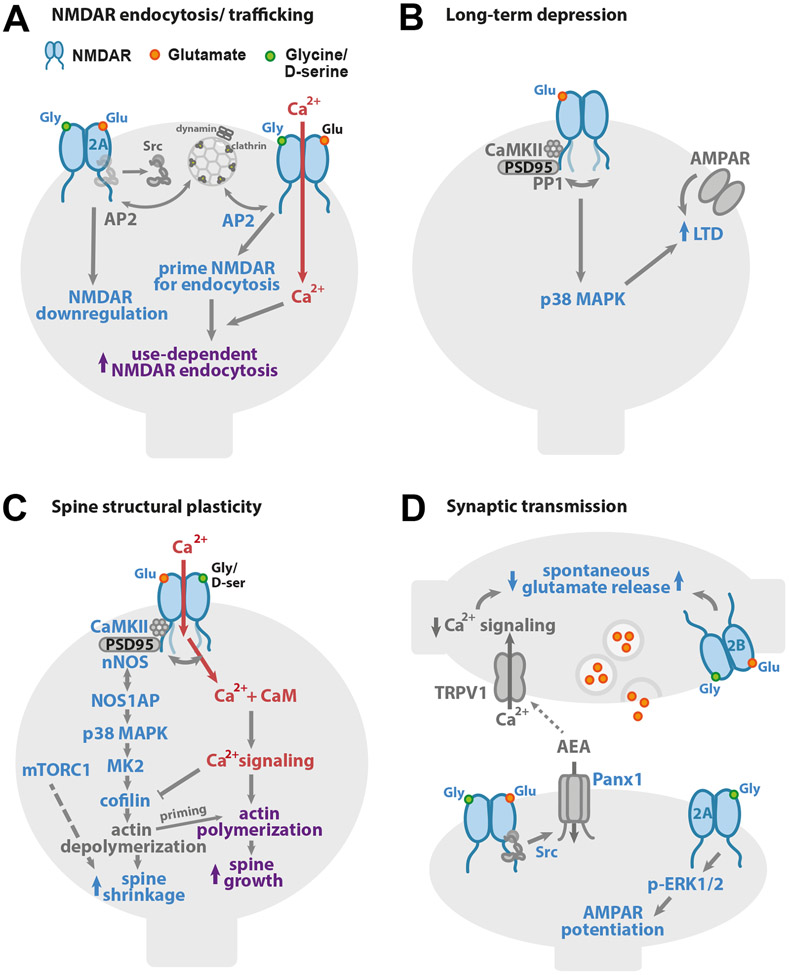
Figure 1. Non-ionotropic NMDAR signaling in neuronal physiology.
(A) Binding of one or both co-agonists drives NMDAR downregulation. On the left, binding of NMDAR agonist and co-agonist drives dephosphorylation of GluN1 Y837 and GluN2A Y842, even when NMDAR ion flux is inhibited, leading to downregulation of the number of functional NMDARs. On the right, high concentrations of glycine (>10 μM) increase the interaction with AP2 and prime the NMDAR for dynamin-dependent endocytosis. (B) Glutamate binding to the NMDAR is sufficient to drive long-term depression (LTD) of synaptic strength, independent of ion flux through the NMDAR. p38 MAPK activation is required for LTD induction. Agonist binding to the NMDAR initiates a conformational change which drives the GluN1 C-tails apart and alters its interactions with CaMKII and PP1. (C) Ion flux-independent NMDAR signaling contributes to bidirectional spine structural plasticity. Glutamate binding with little or no ion flux through the NMDAR drives dendritic spine shrinkage via a signaling pathway that includes the interaction of nNOS and NOS1AP, and the activities of nNOS, p38 MAPK, CaMKII, MK2, and cofilin. Furthermore, the activity of mTORC1 supports a requirement for new protein synthesis. Notably, when this non-ionotropic NMDAR signaling pathway is activated in combination with strong calcium influx, it instead drives dendritic spine growth. (D) Non-ionotropic NMDAR signaling plays a role in the regulation of neurotransmitter release and synaptic strength. On the left, ligand binding to postsynaptic NMDARs activates Pannexin-1 channels through Src. Opening of Pannexin-1 drives clearance of AEA, a ligand for presynaptic TRPV1, from the synaptic cleft. With decreased AEA, TRPV1 closes, reducing calcium available for spontaneous release of glutamate. On the right bottom, in a second mechanism, co-agonist binding to GluN2A-containing postsynaptic NMDARs activates ERK1/2 and increases synaptic strength. On the right top, activation of presynaptic NMDARs by agonist, or both agonist and co-agonist, enhances spontaneous release of glutamate in an ion flux-independent manner.