Abstract
Introduction
Periodontitis, as a chronic, multifactorial inflammatory disease, has complex relationships with other diseases and ultimately with well-being. The aim of this cross-sectional study was to investigate the association between self-report periodontitis, as measured with the recently developed and validated modified Periodontal Screening Score (mPESS), and oral health-related quality of life (OHRQol) in a large population-based sample derived from the French NutriNet-Santé e-cohort.
Methods
The sample was composed of 32,714 adults (75.5% women) with a mean age of 48.8 ± 13.9 years. Periodontitis was assessed based on age, smoking, and oral health status data obtained in 2011–2012, which allowed calculating the mPESS. An mPESS ≥ 5 was used to identify individuals at risk of severe periodontitis (main exposure). OHRQoL was measured with the Oral Health Impact Profile (OHIP-14) (main outcome) and the total score was dichotomized for analysis. Multivariable logistic regression analyses, considering physical health status, dietary and lifestyle confounding variables, were performed.
Results
Overall, 6407 participants (19.6%) were at a high risk of severe periodontitis. A total of 7383 participants (22.6%) presented a relatively poor OHRQoL (OHIP-14 > 8, highest quartile). In the multivariable model, each of the following variables was independently and significantly associated with lower OHRQoL: older age (50–64 years), female sex, obesity, snacking between meals, frequent consumption of soft drinks and sweets/chocolate, risk of severe periodontitis, and having < 20 natural teeth were significantly. An mPESS ≥ 5 showed the highest odds for relatively poor OHRQoL (OR = 3.45; 95% CI 3.21–3.72).
Conclusion
The results support the association between periodontitis and OHRQoL in non-clinical samples. The use of mPESS could be tested in future prevention programs aiming at improving OHRQoL.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11136-022-03215-x.
Keywords: Oral health related quality of life, Oral health, Periodontitis, Questionnaire, Nutrition
Introduction
Health-related quality of life (HRQoL) is an individual’s or a group’s perceived physical and mental health and comfort over time [1]. There is no doubt that oral health, as part of general health, has an impact on the individual’s well-being and quality of life (QoL) overall [1, 2].
Oral health-related quality of life (OHRQoL) is a person-centered outcome based on multidimensional and subjective concepts [3]. It measures the possible impact an oral disease, symptom and/or treatment may have on different dimensions of a person’s life. Previous studies have shown an association between OHRQoL and periodontal diseases, including gingivitis and periodontitis [4–6], but the methodologies were highly heterogeneous. Most of the studies used the Oral Health Impact Profile 14 (OHIP-14) [7], analyzed convenience samples covering an age range of 15–75 years and sample sizes were highly variable, ranging from 24 to 6469 individuals [4]. Further, most of the studies did not address important confounding variables in their analyses, particularly those related to socio-economic characteristics, chronic systemic diseases, oral hygiene or dietary habits. Only a few studies were performed in European countries; a Swedish [8] and a Swiss [9] study included convenience samples of 204 and 215 individuals, respectively. Two larger studies from the UK included community samples and national samples of 3122 [10] and 6318 [11] individuals, respectively. One recent study included a limited number of variables in the multivariable model, none of them related to diet [11]. Nevertheless, the literature has suggested an association between periodontal diseases and OHRQoL [2, 12–16]. Social life, life experiences, and self-confidence are aspects of human nature deeply interwoven with perceptions of happiness and QoL, and all of these aspects are negatively affected by periodontitis [5, 17]. Moreover, periodontitis, as a chronic, multifactorial inflammatory disease, has complex relationships with other diseases, particularly diabetes, hypertension and cardiovascular diseases [18–20], and is linked to many behavioral and lifestyle factors [21], such as smoking habits, psychosocial stress, and nutrition [22, 23]. All of these factors have also been shown to potentially impact QoL and OHRQoL [13, 24–29]. Consequently, research using large non-clinical samples of adults at risk of severe periodontitis are needed, together with control for dietary and other confounding variables, to adequately analyze the relationship between periodontal disease and OHRQoL. The present analysis, which takes into consideration a large array of variables potentially impacting OHRQoL, investigated the association between self-reported periodontitis, as measured with the recently developed modified Periodontal Screening Score (mPESS) and the OHIP-14 score in a large population-based sample derived from the French NutriNet-Santé e-cohort.
Materials and methods
Study design and study population
The ongoing NutriNet-Santé web cohort (www.etude-nutrinet-sante.fr) was launched in 2009 [30]; it was approved by the human subjects participation ethics board of the French Institute for Health and Medical Research and by the National Commission on Informatics and Liberty. NutriNet-Santé is registered at www.clinicaltrials.gov (# NCT03335644). Prior to inclusion, each participant provides an informed consent and an electronic signature.
All participants are adult volunteers (≥ 18 years) with Internet access, having been recruited via multimedia calls. Participation in the e-cohort consists in completing annual questionnaires about socio-demographic, lifestyle, health status, physical activity, anthropometric, and diet-related parameters. On a regular basis during the follow-up, participants also receive questionnaires on particular health- or diet-related topics.
For the present analyses, we selected 39,971 individuals who had enrolled in the cohort between 2009 and 2012 and who had responded to two oral health questionnaires administered in 2011 and 2012 (described below). Participants reporting pregnancy (at the time of the oral health questionnaire completion), being completely edentulous or wearing a complete denture, or with incomplete data from the oral health questionnaires were not eligible. Due to the observational, cross-sectional nature of the study, the STROBE checklist was used as a reporting guideline [31].
Study outcome assessment
In the present study, the main outcome variable was OHRQoL and it was measured using the OHIP-14 [7]. This is one of the most widely used measures in the domain of OHRQoL, particularly in epidemiological studies assessing the impact of periodontal diseases on various health outcomes [32]. It represents the short version of the OHIP-49, originally developed by Slade et al. in 1994 [33], which provides an evaluation of how various aspects of oral health might affect the physical, psychological, and social aspects of the individual’s life [2, 34]. This questionnaire has been translated and validated for use with French-speaking adults [35]. Based on 14 self-report items grouped in 7 dimensions, the OHIP-14 measures functional limitations, physical pain, psychological discomfort, physical disability, psychological disability, social disability, and handicap. The responses are recorded on a Likert scale with values ranging from 0 to 4, as follows: never (score 0), hardly ever (score 1), occasionally (score 2), fairly often (score 3), and very often (score 4). The higher the average value on each of the 7 dimensions, the more negative the impact of one’s oral health on their QoL. The cumulative OHIP-14 score ranges from 0 (best OHRQoL) to 56 (worst OHRQoL), and is calculated by summing up the ordinal values for the 14 items. For each domain, scores can range from 0 to 8. Higher OHIP-14 scores indicate worse OHRQol and lower OHIP-14 scores indicate better OHRQol.
Exposure assessment
Periodontitis was the main exposure in this analysis. This variable was obtained using the French version of the Centers for Disease Control and Prevention and the American Academy of Periodontology (CDC/AAP) instrument [36], integrated in the oral health questionnaires, self-administered in 2011 and 2012. In total, n = 102988 NutriNet-Santé participants received and n = 39971 completed these two questionnaires on a voluntary basis. The CDC/AAP tool allowed us to calculate the modified Periodontal Screening Score, namely the mPESS, based on four specific questions about oral status, plus age and smoking status (Supplemental Table S1) [37]. The mPESS was validated against a clinical diagnosis of periodontitis and was shown to be an accurate self-report tool, with an mPESS ≥ 5 being associated with the highest specificity and sensitivity to detect individuals suffering from severe periodontitis (area under the ROC = 0.815; mPESS sensitivity: 71.3%; specificity: 79.5%) [37, 38]. As a supplemental oral health exposure, we considered the number of natural teeth, dichotomized as < or ≥ 20 teeth [39].
Covariate assessment
Baseline socio-demographic, lifestyle, and health-related variables were also considered as co-exposures. These were obtained from validated questionnaires [40, 41] and included: age, sex, anthropometric measures [based on self-reported weight and height, which allowed the calculation of body mass index (BMI)], education, marital status, socio-professional category, household income, smoking status, mean daily physical activity, mean daily alcohol consumption [38], oral health-related dietary habits (i.e., frequency of snacking, sweets/candy consumption and soft drinks intake), and health status (prevalent diabetes, cancer, and/or major cardiovascular diseases).
Educational level was categorized as high school degree or less, undergraduate or graduate degree. Socio-professional categories included manual workers, administrative staff, self-employed/intermediate-skill professions and executive staff. Household income included 4 monthly revenue categories in Euros (< 1200; 1200–1799; 1800–2699; and ≥ 2700) plus one “not reported” category. Marital status was categorized into married/in a couple or single/divorced/widowed.
Smoking status was categorized as current smoker (every day or occasionally), former or never smoker. Physical activity was modeled as three categories: < 30 min walking/day; between 30and 59 min walking/day; and ≥ 60 min walking/day [20, 38]. Alcohol consumption was obtained from 24-h dietary records completed on 3 non-consecutive days and was expressed in g ethanol/day; the variable was dichotomized according to a sex-specific threshold (>= 20 g/day for women, >= 30 g/day for men), as previously reported [38]. Frequency of snacking between meals and consumption of candy, sweets, chocolate and soft drinks were evaluated by specific questions included in the oral health questionnaires (response options ranging from four times/day to never). Self-reported information about prevalent major cardiovascular disease (myocardial infarction, stroke, and acute coronary syndrome), cancer (any type except basal cell carcinoma) and type 1 or type 2 diabetes was obtained from health status questionnaires; these data were validated using hospital records, reported treatment, and linkage with national medico-administrative databases.
Statistical analyses
Descriptive analyses were used to evaluate OHRQoL in the full sample; results are reported as frequency (%) of OHIP-14 “fairly often or very often” responses and as mean values (standard deviation, SD). Next, given the absence of an established cutoff, the sample was divided into two groups based on the highest OHIP-14 total score quartile, identifying individuals with the poorest OHRQoL. Group comparisons were performed regarding socio-demographics, lifestyle, general health, and oral health variables by Chi-squared tests. The Pearson correlation between mPESS and OHIP-14 as continuous variables was also calculated. Interaction by sex was tested since males and females might differ in their reports of OHRQoL [42] and QoL [43].
Multivariable logistic regression analyses were conducted to assess the association between self-reported severe periodontitis (mPESS >= 5) and poor OHRQoL (highest OHIP-14 quartile). First, a partially adjusted model was tested, including age (continuous scale) and sex as covariables (Model 1); then, a fully adjusted model was run including all covariables that reached a significant difference between the two groups in the bivariate analysis (Model 2). To maintain the total sample size, the “I don’t know” and “Not reported” responses were modeled as a separate response category. Adjusted odds ratios (OR) along with 95% confidence intervals (CI) assessed the strength of the associations for each exposure variable. A sensitivity analysis was carried out to assess the relationship between the main exposure and outcome in participants without chronic diseases, which might confound QoL measures. Statistical analyses were carried out with SPSS (IBM Statistics, version 23). All tests were two-sided and a p value < 0.05 was considered statistically significant.
Results
Sample characteristics
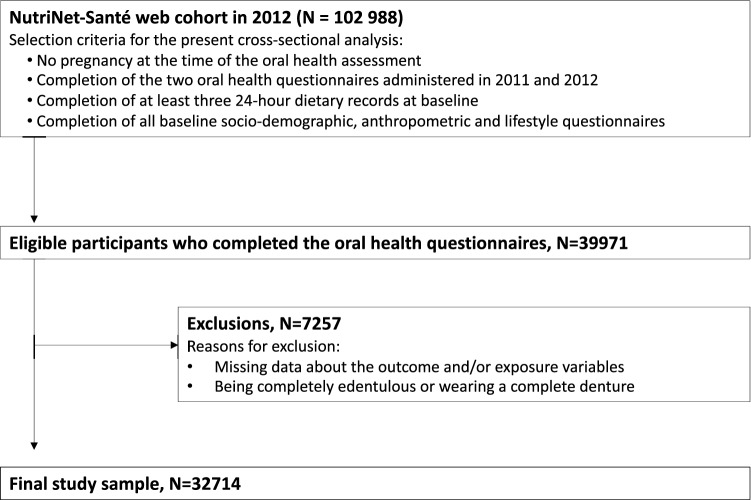
From the sample of 39,971 individuals who completed the two oral health questionnaires, 32,714 adults met the inclusion criteria and were selected for the present analyses (Fig. 1).
Fig. 1.
Flowchart of participant selection from the NutriNet-Santé web cohort
The study sample had a mean age of 48.8±13.9 years; the majority were aged between 26 and 49 years (43.6%) and between 50 and 64 years (37.7%). More than 2/3 of the sample was composed of women. The mean BMI was 23.8±4.3 kg/m2.
According to mPESS, 6407 individuals (19.6%) had self-reported severe periodontitis (4591 females and 1816 males). A significantly higher percentage of individuals with an mPESS ≥ 5 was found among males than females (22.7% vs. 18.6%, respectively; p < 0.0001). Overall, 4899 individuals (15.5%) reported having < 20 natural teeth.
The overall mean OHIP-14 score was 5.63 ± 6.54 (range 0–56), and it was not normally distributed. The items most frequently endorsed were: being self-conscious of oral diseases or having problems with gums and teeth (8.1%), feeling aching in the mouth (4.9%), being uncomfortable when eating (4.3%), feeling tense (3.8%) or embarrassed (3.6%) due to problems with teeth, mouth or dentures (Table 1). The OHIP-14 dimensions that were the most frequently endorsed were physical pain and psychological discomfort. A significant positive correlation was found between the mPESS and the OHIP-14 values (Pearson r: 0.25; p < 0.0001), with a significant quantitative interaction by sex (p < 0.0001). Overall, 7383 participants (22.6%) had an OHIP-14 >8 (highest quartile cutoff) and were considered to have a low OHRQoL.
Table 1.
OHIP-14 questions and dimension scores in the full sample, 2009–2012, NutriNet-Santé web cohort, France (n = 32,714)
| OHIP-14 questions | OHIP-14 dimensions | |||
|---|---|---|---|---|
| Item | Frequency of reporting “fairly/very often” | Mean value (SD) | Dimension | Mean value (SD) |
| Having trouble pronouncing words | 0.6% | 0.11 (0.42) | Functional limitations | 0.32 (0.80) |
| Experiencing worsened sense of taste | 1.0% | 0.22 (0.50) | ||
| Feeling painful aching in the mouth | 4.9% | 1.09 (0.88) | Physical pain | 1.76 (1.57) |
| Being uncomfortable to eat | 4.3% | 0.67 (0.92) | ||
| Being self-conscious | 8.1% | 0.97 (1.02) | Psychological discomfort | 1.48 (1.71) |
| Feeling tense | 3.8% | 0.51 (0.86) | ||
| Avoiding some food—having an unsatisfactory diet | 3.0% | 0.47 (0.82) | Physical disability | 0.58 (1.08) |
| Interrupting meals | 0.4% | 0.11 (0.41) | ||
| Difficulty relaxing | 1.0% | 0.22 (0.58) | Psychological disability | 0.84 (1.28) |
| Feeling embarrassed | 3.6% | 0.62 (0.87) | ||
| Being irritable | 0.9% | 0.22 (0.57) | Social disability | 0.31 (0.84) |
| Difficulty doing usual tasks | 0.4% | 0.09 (0.38) | ||
| Perceiving life as less satisfying | 1.8% | 0.25 (0.65) | Handicap | 0.32 (0.85) |
| Feeling completely unable to function | 0.2% | 0.07 (0.32) | ||
| Total scorea | – |
5.63 (6.54) range (0–56) |
||
OHRQol Oral health-related quality of life, OHIP-14 Oral Health Impact Profile
aThe OHIP-14 score range goes from 0 (best OHRQoL) to 56 (worst OHRQoL)
Association between self−reported periodontitis and OHRQoL in the full sample and by sex
All socio−demographic, lifestyle, general health, and oral health variables except for alcohol intake were significantly different by OHRQoL status [OHIP-14 ≤ 8 (good/average OHRQoL) versus OHIP >8 (low OHRQoL] (Table 2).
Table 2.
Socio-demographic, lifestyle and health status characteristics for the total study sample and by group of individuals with a total OHIP-14 score ≤ 8 versus those with OHIP-14 > 8 (4th quartile corresponding to poorest OHRQoL)
| Variable | Total sample (n = 32,714) | Individuals with OHIP score ≤ 8 (n = 25,331) | Individuals with OHIP score > 8 (n = 7383) | Bivariate analysis P value |
|---|---|---|---|---|
| Socio-demographic variables | ||||
| Age category [n (%)] | < 0.0001 | |||
| 18–25 | 1709 (5.2) | 1383 (5.5) | 326 (4.4) | |
| 26–49 | 14,247 (43.5) | 11,276 (44.5) | 2971 (40.2) | |
| 50–64 | 12,346 (37.8) | 9288 (36.7) | 3058 (41.4) | |
| >= 65 | 4412 (13.5) | 3384 (13.4) | 1028 (13.9) | |
| Sex [n (%)] | < 0.0001 | |||
| Female | 24,719 (75.6) | 18,822 (74.3) | 5897 (79.9) | |
| Male | 7995 (24.4) | 6509 (25.7) | 1486 (20.1) | |
| Marital status [n (%)] | < 0.0001 | |||
| Married, living in a couple | 24,004 (73.4) | 18,785 (74.2) | 5219 (70.7) | |
| Single, divorced, widowed | 8710 (26.6) | 6546 (25.8) | 2164 (29.3) | |
| Educational level [n (%)] | < 0.0001 | |||
| High school or less | 10,460 (32.0) | 7898 (31.2) | 2562 (34.7) | |
| Undergraduate degree | 11,151 (34.1) | 8558 (33.8) | 2593 (35.1) | |
| Graduate degree | 10,867 (33.2) | 8694 (34.3) | 2173 (29.4) | |
| Other/not reported | 236 (0.7) | 181 (0.7) | 55 (0.7) | |
| Household income (€/month) [n (%)] | < 0.0001 | |||
| < 1200 | 2668 (8.2) | 1894 (7.5) | 774 (10.5) | |
| 1200–1799 | 3339 (10.2) | 2491 (9.8) | 848 (11.5) | |
| 1800–2699 | 4960 (15.2) | 3748 (14.8) | 1212 (16.4) | |
| ≥ 2700 | 18,433 (56.3) | 14,552 (57.4) | 3881 (52.6) | |
| Not reported | 3314 (10.1) | 2646 (10.4) | 668 (9.0) | |
| Socio-professional category [n (%)] | < 0.0001 | |||
| Manual workers/farmers | 869 (2.7) | 635 (2.5) | 234 (3.2) | |
| Self-employed/artisan/merchant/entrepreneur/intermediate-skills profession | 10,227 (31.3) | 7880 (31.1) | 2347 (31.8) | |
| Office work/administrative staff | 8539 (26.1) | |||
| Intellectual profession/executive staff | 11,848 (36.2) | 9437 (37.3) | 2411 (32.7) | |
| Not reported | 1231 (3.7) | 991 (3.9) | 240 (3.3) | |
| Lifestyle behaviors | ||||
| Physical activity | 0.002 | |||
| < 30 min of walking/day | 6840 (20.9) | 5184 (20.5) | 1656 (22.5) | |
| ≥ 30 min but < 60 min of walking/day | 6797 (20.8) | 5313 (21.0) | 1484 (20.1) | |
| ≥ 60 min of walking/day | 14,620 (44.7) | 11,391 (45.0) | 3229 (43.7) | |
| Not reported | 4457 (13.6) | 3443 (13.6) | 1014 (13.7) | |
| Smoking status and frequency [n (%)] | < 0.0001 | |||
| Yes, every day | 2951 (9.0) | 2081 (8.2) | 870 (11.8) | |
| Yes, occasionally | 1321 (4.0) | 1032 (4.1) | 289 (3.9) | |
| Former smoker | 11,761 (36.0) | 8982 (35.5) | 2779 (37.6) | |
| Never smoked | 16,681 (51.0) | 13,236 (52.3) | 3445 (46.7) | |
| Daily alcohol consumption above the sex-specific thresholda [n (%)] | 0.075 | |||
| Yes | 2793 (8.5) | 2125 (8.4) | 668 (9.0) | |
| No | 29,921 (91.5) | 23,206 (91.6) | 6715 (91.0) | |
| Frequency of snacking between meals [n (%)] | < 0.0001 | |||
| 4 times/day | 27 (0.1) | 13 (0.1) | 14 (0.2) | |
| 1–3 times/day | 887 (2.7) | 566 (2.2) | 321 (4.3) | |
| < once/day | 12,259 (37.5) | 9233 (36.4) | 3026 (41.0) | |
| Never | 19,541 (59.7) | 15,519 (79.4) | 4022 (54.5) | |
| Frequency of consumption of chocolate, candies, and sweets [n (%)] | < 0.0001 | |||
| 4 times/day | 227 (0.7) | 142 (0.6) | 85 (1.2) | |
| 1–3 times/day | 8993 (27.5) | 6707 (26.5) | 2286 (31.0) | |
| < once/day | 21,039 (64.3) | 16,542 (65.3) | 4497 (60.9) | |
| Never | 2455 (7.5) | 1940 (7.7) | 515 (7.0) | |
| Frequency of consumption of soft drinks [n (%)] | < 0.0001 | |||
| 4 times/day | 169 (0.5) | 114 (0.5) | 55 (0.7) | |
| 1–3 times/day | 4546 (13.9) | 3463 (13.6) | 1083 (14.7) | |
| < once/day | 15,771 (48.2) | 12,187 (48.1) | 3584 (48.5) | |
| Never | 12,228 (37.4) | 9567 (37.8) | 2661 (36.0) | |
| Physical health status | ||||
| BMI category, kg/m2 [n (%)] | < 0.0001 | |||
| < 25 | 22,903 (70.0) | 17,832 (70.4) | 5071 (68.7) | |
| 25–29 | 7140 (21.8) | 5565 (22.0) | 1575 (21.3) | |
| >= 30 | 2671 (8.2) | 1934 (7.6) | 737 (10.0) | |
| Diabetes (Type I or II, prevalent cases) [n (%)] | < 0.0001 | |||
| Yes | 731 (2.2) | 526 (2.1) | 205 (2.8) | |
| No | 31,983 (97.8) | 24,805 (97.9) | 7178 (97.2) | |
| Cardiovascular diseases (prevalent cases)b [n (%)] | 0.013 | |||
| Yes | 468 (1.4) | 340 (1.3) | 128 (1.7) | |
| No | 32,246 (98.6) | 24,991 (98.7) | 7255 (99.3) | |
| Cancers (any type or organ, prevalent cases)c [n (%)] | 0.029 | |||
| Yes | 2174 (6.6) | 1642 (6.5) | 532 (7.2) | |
| No | 30,540 (93.4) | 23,689 (93.5) | 6851 (92.8) | |
| Periodontal health variables | ||||
| mPESS (modified Periodontal Screening Score) | < 0.0001 | |||
| mPESS ≥ 5 | 6407 (19.6) | 3707 (14.6) | 2700 (36.6) | |
| mPESS < 5 | 26,307 (80.4) | 21,624 (85.4) | 4683 (63.4) | |
| Number of natural teeth [n (%)] | < 0.0001 | |||
| < 20 teeth | 4899 (15.0) | 2930 (11.6) | 1,969 (26.7) | |
| ≥ 20 teeth | 26,735 (81.7) | 21,623 (85.4) | 5112 (69.2) | |
| I don’t know | 1080 (3.3) | 778 (3.1) | 302 (4.1) | |
aThreshold used: ≥ 20 g/day of ethanol for women, ≥ 30 g/day of ethanol for men [38]
bIncluding myocardial infarction, stroke, and acute coronary syndrome
cExcluding basal cell carcinoma
In the full sample, the multivariable analysis adjusted for age and sex only (Model 1) showed a number of significant associations with poor OHRQoL; particularly, mPESS ≥ 5 increased the odds of having OHIP-14 > 8 (OR = 3.95; 95% CI 3.7–4.32). Older age, female sex, being overweight or obese, being a current or former smoker, living alone, suffering from diabetes or cardiovascular diseases, snacking between meals, frequently consuming candy, sweets, chocolate or soft drinks were also associated with higher odds of poor OHRQoL (Table 3). Conversely, daily physical activity of > 30 min of walking, monthly household income > 2700€, and higher socio-professional categories were found to be protective factors regarding OHRQoL. After a full adjustment (Model 2), severe periodontitis in the total sample was associated with higher odds (OR = 3.45, 95% CI 3.21–3.72) of poor OHRQoL. Having < 20 natural teeth in the oral cavity was also associated with higher odds (OR = 1.48, 95% CI 1.29–1.71) of poor OHRQoL. Some lifestyle habits were associated with low OHRQoL, such as smoking (OR = 1.24, 95% CI 1.06–1.46), snacking between meals (OR = 1.64, 95% CI 1.42–1.90), and consuming chocolate, candies, and sweets (OR = 1.23, 95% CI 1.16–1.31). Overweight or obese or participants had a poorer OHRQoL as compared to participants with a BMI < 25. Older participants had a greater likelihood of reduced OHRQoL compared to their younger counterparts.
Table 3.
Logistic regression analysis of the association between main and supplementary exposures (likelihood of severe periodontitis, number of natural teeth, socio-demographic, general health, and lifestyle variables) and odds of poorer oral health-related quality of life (OHIP-14 > 8, 4th quartile) in the full sample, 2009–2012, NutriNet-Santé web cohort, France (n = 32,714)
| Variable | Full sample (n = 32,714) | |
|---|---|---|
| Adjusted OR (95% CI) Model 1a |
Adjusted OR (95% CI) Model 2b |
|
| Age category | ||
| 18–25 | Reference | Reference |
| 26–49 | 1.14 (1.01–1.30) | 1.52 (1.28–1.81) |
| 50–64 | 1.44 (1.27–1.64) | 1.68 (1.52- 1.85) |
| ≥ 65 | 1.43 (1.24–1.65) | 1.22 (1.12–1.34) |
| Sex | ||
| Female | 1.42 (1.33–1.52) | 1.35 (1.26–1.45) |
| Male | Reference | Reference |
| Marital status | ||
| Married, living in a couple | Reference | Reference |
| Single, divorced, widowed | 1.19 (1.13–1.27) | 1.04 (0.96–1.11) |
| Educational level | ||
| High school or less | Reference | Reference |
| Undergraduate degree | 0.92 (0.68–1.25) | 1.03 (0.74–1.42) |
| Graduate degree | 0.97 (0.72–1.32) | 0.97 (0.70–1.34) |
| Other/not reported | 1.13 (0.83–1.54) | 1.02 (0.74–1.41) |
| Household income (€/month) | ||
| < 1200 | Reference | Reference |
| 1200–1799 | 0.53 (0.51–0.65) | 0.62 (0.54–0.70) |
| 1800–2699 | 0.73 (0.65–0.82) | 0.77 (0.69–0.88) |
| ≥ 2700 | 0.77 (0.69–0.82) | 0.81 (0.72–0.90) |
| Other/not reported | 0.93 (0.84–1.02) | 0.91 (0.82–1.00) |
| Socio-professional category | ||
| Manual workers/farmers | Reference | Reference |
| Self-employed artisan/merchant / entrepreneur/intermediate-skills profession | 1.06 (0.91–1.24) | 1.03 (0.86–1.24) |
| Office work/administrative staff | 0.70 (0.56–0.86) | 0.81 (0.64–1.02) |
| Intellectual profession/executive staff | 0.84 (0.72–0.98) | 0.91 (0.75–1.08) |
| Other/not reported | 0.95 (0.81–1.11) | 0.97 (0.81–1.17) |
| Physical activity | ||
| < 30 min of walking/day | Reference | Reference |
| ≥ 30 min of walking/day | 0.90 (0.85–0.95) | 0.91 (0.86–0.96) |
| Smoking status and frequency | ||
| Every day | 1.64 (1.51–1.80) | 1.24 (1.06–1.46) |
| Occasionally | 1.40 (1.27–1.53) | 1.12 (0.97–1.29) |
| Former smoker | 1.45 (1.24–1.69) | 1.10 (1.01–1.21) |
| Never smoked | Reference | Reference |
| Daily alcohol consumption above the sex-specific thresholda | ||
| Yes | 1.11 (1.00–1.22) | 1.03 (0.94–1.14) |
| No | Reference | Reference |
| Frequency of snacking between meals | ||
| 1–4 times/day | 2.02 (1.76–2.32) | 1.64 (1.42–1.90) |
| Less than once/day or never | Reference | Reference |
| Frequency of consumption of chocolate, candies, sweets | ||
| 1–4 times/day | 1.27 (1.20–1.34) | 1.23 (1.16–1.31) |
| Less than once/day or never | Reference | Reference |
| Frequency of consumption of soft drinks | ||
| 1–4 times/day | 1.20 (1.11–1.29) | 1.15 (1.06–1.24) |
| Less than once/day or never | Reference | Reference |
| BMI category (kg/m2) | ||
| < 25 | Reference | Reference |
| 25–29 | 1.32 (1.20–1.44) | 1.15 (1.04–1.27) |
| >= 30 | 1.29 (1.17–1.43) | 1.21 (1.09–1.35) |
| Prevalent diabetes (Type I or type II) | ||
| Yes | 1.35 (1.15–1.6) | 1.11 (0.92–1.32) |
| No | Reference | Reference |
| Prevalent major cardiovascular disease | ||
| Yes | 1.32 (1.07–1.62) |
1.19 (0.95–1.49) Reference |
| No | Reference | |
| Prevalent cancer | ||
| Yes | 1.03 (0.93–1.14) | 0.99 (0.88–1.11) |
| No | Reference | Reference |
| mPESS (modified Periodontal Screening Score) | ||
| mPESS ≥ 5 | 3.95 (3.70–4.32) | 3.45 (3.21–3.72) |
| mPESS < 5 | Reference | Reference |
| Number of natural teeth [n (%)] | ||
| < 20 teeth | 1.65 (1.44–1.89) | 1.48 (1.29–1.71) |
| ≥ 20 teeth | Reference | Reference |
aModel 1 is adjusted for age (continuous variable) and sex
bModel 2 is adjusted for age (continuous variable), sex, sociodemographic variables, lifestyle habits, physical health status, and periodontal health
The sex-specific results were largely consistent with the main results, however differences in magnitude emerged (Table 4).
Table 4.
Logistic regression analysis of the association between likelihood of severe periodontitis and oral health-related quality of life (OHIP-14 > 8, 4th quartile) in women and men, 2009–2012, NutriNet-Santé web cohort, France
| Exposure | Women (n = 24,719) | Men (n = 7995) | ||
|---|---|---|---|---|
| Adjusted OR (95% CI) Model 1a |
Adjusted OR (95% CI) Model 2b |
Adjusted OR (95% CI) Model 1a |
Adjusted OR (95% CI) Model 2b |
|
| mPESS (modified Periodontal Screening Score) | ||||
| mPESS ≥ 5 | 3.95 (3.65–4.27) | 3.46 (3.18–3.76) | 4.42 (3.84–5.09) | 3.88 (3.33–4.51) |
| mPESS < 5 | Reference | Reference | Reference | Reference |
| Number of natural teeth [n (%)] | ||||
| < 20 teeth | 1.59 (1.36–1.86) | 1.44 (1.22–1.69) | 1.86 (1.40–2.47) | 1.77 (1.31–2.38) |
| ≥ 20 teeth | Reference | Reference | Reference | Reference |
aModel 1 is adjusted for age (continuous variable)
bModel 2 is adjusted for sociodemographic variables, lifestyle habits, physical health status and periodontal health
Due to the inverse association between QoL measures and chronic illness [44, 45], a sensitivity analysis was performed in a subgroup of n = 29,510 individuals after excluding all participants with prevalent cancer, major cardiovascular diseases and/or diabetes. The results largely replicated the main findings, showing that periodontitis (mPESS ≥ 5) remained significantly associated with OHIP-14 > 8 (fully adjusted OR = 3.69; 95% CI 3.42–3.97) (data not tabulated).
Discussion
This population-based cross-sectional study investigated the association between self-reported severe periodontitis and OHRQoL, taking into consideration socio-demographic, dietary, lifestyle, and general health status variables. The results, based on a large, heterogeneous sample of French adults enrolled in the NutriNet-Santé web cohort, showed that the risk of severe periodontitis, assessed via a new screening tool—the mPESS—was strongly linked to OHRQoL. It is important to note that an mPESS ≥ 5 was independently associated with a 3.4 times increased odds of having poorer OHRQoL. The direction and significance of the associations were consistent across sex, yet the effect sizes were somewhat stronger in males than in females. In addition, the results remained largely unchanged after excluding individuals with chronic diseases.
In accordance with previous reports, the most affected OHRQoL domains in the NutriNet-Santé e-cohort were physical pain, psychological discomfort and disabilities [4, 9, 10, 46, 47]. Assessing the impact of oral health/illness on QoL (i.e., OHRQoL) is complex due to the presence of multiple exposures beyond the oral cavity, which can be interdependent. For instance, the number of teeth can affect masticatory capacity, which may lead to changes in dietary habits and ultimately may have consequences regarding BMI status and general health [48–50]. Moreover, some oral diseases, including periodontitis, have a multifactorial etiology that includes physical health status and behavioral risk factors, such as smoking, alcohol consumption, poor dietary habits, physical inactivity, and diabetes [38, 51–54]. Most of these factors were assessed in the NutriNet-Santé e-cohort allowing for an independent evaluation of OHRQoL related to periodontitis. This represents the main novelty and strength of the study, in addition to using a relatively new periodontitis screening tool. While confirming some previous results on the impact of periodontal diseases on OHRQoL [4], our analysis showed that BMI, physical activity, lifestyle and nutritional habits are associated with OHIP-14.
Another strength of the present study was the size and diversity of the sample, which is unique in the literature, and would be hard to attain using clinical approaches, which are time-consuming and not easily applicable in large-scale epidemiological studies [36, 37, 55, 56]. Indeed, the present results showed for the first time that a self-report-based tool developed to identify individuals likely to suffer from severe periodontitis, the mPESS [37, 38], is strongly associated with the OHIP-14 score. The mPESS calculated among the participants in the NutriNet-Santé e-cohort showed similar sensitivity and specificity as those in the original sample (PESS sensitivity: 78.9%; specificity: 74.8%%) [38]. The mPESS takes into account 4 signs and symptoms of periodontitis plus age and current smoking status, which are known risk factors for periodontitis [57]. Thus, this easily employed tool allows for the assessment of multidimensional aspects of periodontitis, and could better capture their impact on OHRQoL compared to other oral health measures, such as epidemiological indices, e.g., the community periodontal index (CPI), and clinical parameters, e.g., clinical attachment level [4]. Moreover, at the present time in which the Covid-19 pandemic has negatively impacted patients’ attitudes (e.g., fear of getting infected with the SARS-Cov-2 virus in the dentist’s office) and access to dental care [58–61], the self-reported evaluation of periodontitis may be a valuable supporting tool to reinforce prevention and treatment.
Tooth loss is one of the worst types of damage to oral health; the main reasons for tooth loss in adulthood are periodontitis and caries. Tooth loss can cause both esthetic and functional problems [62, 63], and it has been shown to be associated with impaired OHRQoL [63, 64]. The location and distribution of missing teeth also affect the degree of this impairment [65], with a negative impact that increases sharply once the number of remaining teeth drops below 20 [65]. Consistent with the literature, the present study showed that having fewer than 20 teeth in the oral cavity increased the odds of low OHRQoL by 48%. However, it must be noted that both periodontal status and number of teeth were self-reported and thus comparisons with previous studies employing parameters assessed clinically (e.g., periodontal probing, clinical attachment level measurements, gingival inflammation evaluation) require a cautious interpretation. Different measures could generate different data, partly explaining the heterogeneity of the findings [4, 8, 9].
Regarding the socio-demographic variables (modeled as supplementary exposures), poorer OHRQoL appeared to be associated with female sex, older age, and decreased household income. These results are in agreement with previous studies [66–68], although contrasting results have also been reported [69, 70], raising issues about the relative weight of these factors.
Compromised general health, comorbidities, and medication use are also supported as conditions that can enhance the effects that oral pathologies might have on OHRQoL, as seen in diabetic or overweight individuals [29, 71]. In the present study, diabetes, cancer, and major cardiovascular diseases were not significantly associated with poor OHRQoL, whereas a BMI > 25 was associated with an increased odds of having a reduced OHRQoL (OHIP-14 score > 8: OR = 1.15 (95% CI 1.04–1.27) for overweight and 1.21 (95% CI 1.09–1.35) for obese individuals).
Another important finding of the present study was that the OHIP-14 score appeared to be independently related to some unhealthy dietary habits, such as snacking between meals (increased odds by 64%), as well as intake of soft drinks (increased odds by 15%) and sweets/chocolate consumption more than once per day (increased odds by 23%). These dietary habits are known to impact general and oral health (i.e. odds of caries). Andreeva et al. [24] found that adherence to French dietary guidelines, as assessed by the modified Program National Nutrition Santé-Guidelines Score (PNNS-GS), was positively yet modestly correlated with OHRQoL assessed with the Geriatric Oral Health Assessment Index (GOHAI). In another study, lower OHIP-14 global score was found in patients with eating disorders, who presented with more functional limitations, physical disability, psychological and social disability, and handicap [72]. However, to our knowledge, no previous periodontitis study has taken into consideration the role of nutritional habits in OHRQoL as measured with the OHIP-14 in a sample derived from the general population.
The pros and cons of web-based questionnaires have been widely discussed elsewhere [20, 24, 38, 73, 74]. Next, as a cross-sectional investigation, no conclusion about causality between periodontitis and OHRQoL can be drawn. The study sample, although recruited from the French general population, cannot be considered fully representative, and care must be taken when generalizing the findings [74]. In fact, the proportions of women, relatively well-educated individuals and those who are married/cohabiting, are larger in the cohort compared with the corresponding national figures [74]. However, we have also observed that the cohort exhibited geographical and socio-demographic diversity, including volunteers belonging to typically under-represented subgroups in traditional surveys. Next, given the Internet-based design of NutriNet-Santé, and the fact that older age has been associated with reduced Internet access [75], and with increased risk of dental enamel erosion and oral health problems [76], we could speculate that the findings might be somewhat biased owing to the potentially low representation in the sample of individuals with compromised dental status. Nonetheless, the estimated prevalence of severe periodontitis in the cohort was similar to the national estimate [37, 38, 77]. Next, prior oral health research in the NutriNet-Santé cohort revealed socio-demographic and lifestyle differences between those who did and did not meet the analysis eligibility criteria [73]. Finally, in an effort to prevent over-adjustment of the statistical models, only major comorbidities with a good level of evidence in terms of association with periodontitis were retained as covariates. However, it cannot be excluded that other comorbidities might also have an impact on periodontitis risk. Similarly, in this epidemiological cohort we could not assess clinical parameters such as dental caries and/or malocclusion, which may contribute to OHQoL.
Conclusion
The mPESS, as a new, validated indicator of the likelihood of severe periodontitis, was shown to be strongly associated with a lower OHRQoL across sex and age in a large non-clinical sample of adults. These results suggest that mPESS could be tested in future prevention programs aiming at improving oral health-related quality of life.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors thank the following individuals for their technical contribution to the NutriNet-Santé study: thi Hong Van Duong, Régis Gatibelza, Jagatjit Mohinder, and Aladi Timera (computer scientists); Fabien Szabo de Edelenyi, Ph.D. (data management supervisor); Nathalie Arnault, Julien Allegre, Laurent Bourhis, Nicolas Dechamp (data managers/statisticians); Merveille Kouam (health event validator); Cédric Agaesse, Rebecca Lutchia, Alexandre De Sa (dietitians); Maria Gomes (participant support); Younes Esseddik (IT supervisor); and Nathalie Druesne-Pecollo, Ph.D. (operations coordinator). Finally, the authors thank all volunteers in the NutriNet-Santé cohort.
Author contributions
LJ and ZH contributed to data analyses, data interpretation, and drafting the manuscript. SH and MT developed and implemented the NutriNet-Santé web cohort. CJ, SH, MT, and VAA coordinated the data collection and participated in data interpretation. PB contributed to study design and data interpretation, and provided theoretical and methodological guidance. MCC contributed to study design, data analysis, data interpretation, literature review and drafting the manuscript. VAA coordinates the oral health working group within NutriNet-Santé; she contributed to data analysis, data interpretation, and drafting the manuscript. All authors read and critically revised the manuscript for important intellectual content. All authors approved the final version of the manuscript and its submission.
Funding
The NutriNet-Santé study is supported by the French Ministry of Solidarity and Health, the National Agency for Public Health (Santé Publique France), the National Institute for Health and Medical Research (INSERM), the National Research Institute for Agriculture, Food and Environment (INRAE), the National Conservatory of Arts and Crafts (CNAM), the Center for Epidemiological Research and Statistics (CRESS) and Sorbonne Paris Nord University. The funders had no role in the design of the study; in the collection, analysis, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Declarations
Conflict of interest
All authors have no relevant financial or non-financial interests to disclose.
Ethical approval
The NutriNet-Santé study was approved by the human subjects participation ethics board of the French Institute for Health and Medical Research and by the National Commission on Informatics and Liberty. NutriNet-Santé is registered at www.clinicaltrials.gov (# NCT03335644).
Consent to participate
Prior to inclusion in the e-cohort, each participant provides an informed consent and an electronic signature.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Centers for Disease Control and Prevention. Health-Related Quality of Lige (HRQOL). (2021). Retrieved 29 June 2022, from https://www.cdc.gov/hrqol/index.htm#:~:text=Health%2Drelated%20quality%20of%20life%20(HRQOL)%20is%20an%20individual's,role%20in%20public%20health%20practice
- 2.Buset SL, Walter C, Friedmann A, Weiger R, Borgnakke WS, Zitzmann NU. Are periodontal diseases really silent? A systematic review of their effect on quality of life. Journal of Clinical Periodontology. 2016;43(4):333–344. doi: 10.1111/jcpe.12517. [DOI] [PubMed] [Google Scholar]
- 3.Graziani F, Minenna L, Karapets D, Herrera D, Nisi M, Gennai S, Gabriele M, Discepoli N, Petrini M, Marhl U, Perić M, Adriaen L, Alonso B, Bouchard P, Cardaropoli D, Cavalcanti R, Chackartchi T, Franch-Chillida F, Gianserra R, Guerrero A, Landi L, Masiero S, Mensi M, Moratti P, Oreglia F, Rupe A, Sanchez I, Sforza N, Capri D, Zabalegui I, Sanz M, Tonetti M, Tomasi C. Oral care and quality evaluation: A multicentric study on periodontal treatment. Oral Health & Preventive Dentistry. 2020;18(1):363–371. doi: 10.3290/j.ohpd.a44444. [DOI] [PubMed] [Google Scholar]
- 4.Ferreira MC, Dias-Pereira AC, Branco-de-Almeida LS, Martins CC, Paiva SM. Impact of periodontal disease on quality of life: A systematic review. Journal of Periodontal Research. 2017;52(4):651–665. doi: 10.1111/jre.12436. [DOI] [PubMed] [Google Scholar]
- 5.Needleman I, McGrath C, Floyd P, Biddle A. Impact of oral health on the life quality of periodontal patients. Journal of Clinical Periodontology. 2004;31(6):454–457. doi: 10.1111/j.1600-051X.2004.00498.x. [DOI] [PubMed] [Google Scholar]
- 6.Nisanci Yilmaz MN, Bulut S, Bakirarar B. Impact of stage-grade of periodontitis and self-reported symptoms on oral health-related quality of life. International Journal of Dental Hygiene. 2021;20(2):291–300. doi: 10.1111/idh.12551. [DOI] [PubMed] [Google Scholar]
- 7.Slade GD. Derivation and validation of a short-form oral health impact profile. Community Dentistry and Oral Epidemiology. 1997;25(4):284–290. doi: 10.1111/j.1600-0528.1997.tb00941.x. [DOI] [PubMed] [Google Scholar]
- 8.Andersson P, Hakeberg M, Karlberg G, Ostberg AL. Clinical correlates of oral impacts on daily performances. International Journal of Dental Hygiene. 2010;8(3):219–226. doi: 10.1111/j.1601-5037.2010.00456.x. [DOI] [PubMed] [Google Scholar]
- 9.Aslund M, Pjetursson BE, Lang NP. Measuring oral health-related quality-of-life using OHQoL-GE in periodontal patients presenting at the University of Berne, Switzerland. Oral Health and Preventive Dentistry. 2008;6(3):191–197. [PubMed] [Google Scholar]
- 10.Bernabé E, Marcenes W. Periodontal disease and quality of life in British adults. Journal of Clinical Periodontology. 2010;37(11):968–972. doi: 10.1111/j.1600-051X.2010.01627.x. [DOI] [PubMed] [Google Scholar]
- 11.Masood M, Younis LT, Masood Y, Bakri NN, Christian B. Relationship of periodontal disease and domains of oral health-related quality of life. Journal of Clinical Periodontology. 2019;46(2):170–180. doi: 10.1111/jcpe.13072. [DOI] [PubMed] [Google Scholar]
- 12.Peres MA, Macpherson LMD, Weyant RJ, Daly B, Venturelli R, Mathur MR, Listl S, Celeste RK, Guarnizo-Herreño CC, Kearns C, Benzian H, Allison P, Watt RG. Oral diseases: A global public health challenge. Lancet. 2019;394(10194):249–260. doi: 10.1016/S0140-6736(19)31146-8. [DOI] [PubMed] [Google Scholar]
- 13.Goh V, Hassan FW, Baharin B, Rosli TI. Impact of psychological states on periodontitis severity and oral health-related quality of life. Journal of Oral Science. 2022;64(1):1–5. doi: 10.2334/josnusd.21-0267. [DOI] [PubMed] [Google Scholar]
- 14.Izidoro C, Botelho J, Machado V, Reis AM, Proenca L, Alves R, Mendes JJ. Periodontitis, halitosis and oral-health-related quality of life: A cross-sectional study. Journal of Clinical Medicine. 2021;10(19):4415. doi: 10.3390/jcm10194415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kutsal D, Bilgin Cetin M, Durukan E, Bulut S. Evaluation of the effect of periodontitis on quality of life using Oral-Dental Health-Related Quality of Life-United Kingdom scale. International Journal of Dental Hygiene. 2021;19(3):305–312. doi: 10.1111/idh.12501. [DOI] [PubMed] [Google Scholar]
- 16.Bhargava N, Jadhav A, Kumar P, Kapoor A, Mudrakola DP, Singh S. Oral health-related quality of life and severity of periodontal disease. Journal of Pharmacy and Bioallied Sciences. 2021;13(Suppl 1):S387–S390. doi: 10.4103/jpbs.JPBS_588_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Graziani F, Music L, Bozic D, Tsakos G. Is periodontitis and its treatment capable of changing the quality of life of a patient? British Dental Journal. 2019;227(7):621–625. doi: 10.1038/s41415-019-0735-3. [DOI] [PubMed] [Google Scholar]
- 18.Sanz M, Ceriello A, Buysschaert M, Chapple I, Demmer RT, Graziani F, Herrera D, Jepsen S, Lione L, Madianos P, Mathur M, Montanya E, Shapira L, Tonetti M, Vegh D. Scientific evidence on the links between periodontal diseases and diabetes: Consensus report and guidelines of the joint workshop on periodontal diseases and diabetes by the International Diabetes Federation and the European Federation of Periodontology. Journal of Clinical Periodontology. 2018;45(2):138–149. doi: 10.1111/jcpe.12808. [DOI] [PubMed] [Google Scholar]
- 19.Sanz M, Marco Del Castillo A, Jepsen S, Gonzalez-Juanatey JR, D'Aiuto F, Bouchard P, Chapple I, Dietrich T, Gotsman I, Graziani F, Herrera D, Loos B, Madianos P, Michel JB, Perel P, Pieske B, Shapira L, Shechter M, Tonetti M, Vlachopoulos C, Wimmer G. Periodontitis and cardiovascular diseases: Consensus report. Journal of Clinical Periodontology. 2020;47(3):268–288. doi: 10.1111/jcpe.13189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carra MC, Fessi S, Detzen L, Darnaud C, Julia C, Hercberg S, Touvier M, Andreeva VA, Bouchard P. Self-reported periodontal health and incident hypertension: Longitudinal evidence from the NutriNet-Santé e-cohort. Journal of Hypertension. 2021;39(12):2422–2430. doi: 10.1097/HJH.0000000000002941. [DOI] [PubMed] [Google Scholar]
- 21.Ramseier CA, Woelber JP, Kitzmann J, Detzen L, Carra MC, Bouchard P. Impact of risk factor control interventions for smoking cessation and promotion of healthy lifestyles in patients with periodontitis: A systematic review. Journal of Clinical Periodontology. 2020;47:90–106. doi: 10.1111/jcpe.13240. [DOI] [PubMed] [Google Scholar]
- 22.Dommisch H, Kuzmanova D, Jonsson D, Grant M, Chapple I. Effect of micronutrient malnutrition on periodontal disease and periodontal therapy. Periodontology 2000. 2018;78(1):129–153. doi: 10.1111/prd.12233. [DOI] [PubMed] [Google Scholar]
- 23.Chapple IL, Van der Weijden F, Doerfer C, Herrera D, Shapira L, Polak D, Madianos P, Louropoulou A, Machtei E, Donos N, Greenwell H, Van Winkelhoff AJ, Eren Kuru B, Arweiler N, Teughels W, Aimetti M, Molina A, Montero E, Graziani F. Primary prevention of periodontitis: Managing gingivitis. Journal of Clinical Periodontology. 2015;42(Suppl 16):S71–76. doi: 10.1111/jcpe.12366. [DOI] [PubMed] [Google Scholar]
- 24.Andreeva VA, Kesse-Guyot E, Galan P, Feron G, Hercberg S, Hennequin M, Sulmont-Rosse C. Adherence to national dietary guidelines in association with oral health impact on quality of life. Nutrients. 2018;10(5):527. doi: 10.3390/nu10050527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martinelli LM, Mizutani BM, Mutti A, D’Elia MP, Coltro RS, Matsubara BB. Quality of life and its association with cardiovascular risk factors in a community health care program population. Clinics (São Paulo, Brazil) 2008;63(6):783–788. doi: 10.1590/S1807-59322008000600013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bakri NN, Tsakos G, Masood M. Smoking status and oral health-related quality of life among adults in the United Kingdom. British Dental Journal. 2018;225(2):153–158. doi: 10.1038/sj.bdj.2018.529. [DOI] [PubMed] [Google Scholar]
- 27.Sagtani RA, Thapa S, Sagtani A. Smoking, general and oral health related quality of life—A comparative study from Nepal. Health and Quality of Life Outcomes. 2020;18(1):257. doi: 10.1186/s12955-020-01512-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang ES, Brown SE, Ewigman BG, Foley EC, Meltzer DO. Patient perceptions of quality of life with diabetes-related complications and treatments. Diabetes Care. 2007;30(10):2478–2483. doi: 10.2337/dc07-0499.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Irani FC, Wassall RR, Preshaw PM. Impact of periodontal status on oral health-related quality of life in patients with and without type 2 diabetes. Journal of Dentistry. 2015;43(5):506–511. doi: 10.1016/j.jdent.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 30.Hercberg S, Castetbon K, Czernichow S, Malon A, Mejean C, Kesse E, Touvier M, Galan P. The Nutrinet-Sante Study: A web-based prospective study on the relationship between nutrition and health and determinants of dietary patterns and nutritional status. BMC Public Health. 2010;10:242. doi: 10.1186/1471-2458-10-242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Knottnerus A, Tugwell P. STROBE—A checklist to Strengthen the Reporting of Observational Studies in Epidemiology. Journal of Clinical Epidemiology. 2008;61(4):323. doi: 10.1016/j.jclinepi.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 32.Wong LB, Yap AU, Allen PF. Periodontal disease and quality of life: Umbrella review of systematic reviews. Journal of Periodontal Research. 2021;56(1):1–17. doi: 10.1111/jre.12805. [DOI] [PubMed] [Google Scholar]
- 33.Slade GD, Spencer AJ. Development and evaluation of the Oral Health Impact Profile. Community Dental Health. 1994;11(1):3–11. [PubMed] [Google Scholar]
- 34.Sischo L, Broder HL. Oral health-related quality of life: What, why, how, and future implications. Journal of Dental Research. 2011;90(11):1264–1270. doi: 10.1177/0022034511399918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Allison P, Locker D, Jokovic A, Slade G. A cross-cultural study of oral health values. Journal of Dental Research. 1999;78(2):643–649. doi: 10.1177/00220345990780020301. [DOI] [PubMed] [Google Scholar]
- 36.Eke PI, Dye BA, Wei L, Slade GD, Thornton-Evans GO, Beck JD, Taylor GW, Borgnakke WS, Page RC, Genco RJ. Self-reported measures for surveillance of periodontitis. Journal of Dental Research. 2013;92(11):1041–1047. doi: 10.1177/0022034513505621. [DOI] [PubMed] [Google Scholar]
- 37.Carra MC, Gueguen A, Thomas F, Pannier B, Caligiuri G, Steg PG, Zins M, Bouchard P. Self-report assessment of severe periodontitis: Periodontal screening score development. Journal of Clinical Periodontology. 2018;45(7):818–831. doi: 10.1111/jcpe.12899. [DOI] [PubMed] [Google Scholar]
- 38.Hamdi Z, Detzen L, Fessi S, Julia C, Hercberg S, Czernichow S, Boillot A, Touvier M, Bouchard P, Andreeva VA, Carra MC. Alcoholic beverage consumption, smoking habits, and periodontitis: A cross-sectional investigation of the NutriNet-Santé study. Journal of Periodontology. 2021;92(5):727–737. doi: 10.1002/JPER.20-0192. [DOI] [PubMed] [Google Scholar]
- 39.Hobdell M, Petersen PE, Clarkson J, Johnson N. Global goals for oral health 2020. International Dental Journal. 2003;53(5):285–288. doi: 10.1111/j.1875-595x.2003.tb00761.x. [DOI] [PubMed] [Google Scholar]
- 40.Lassale C, Péneau S, Touvier M, Julia C, Galan P, Hercberg S, Kesse-Guyot E. Validity of web-based self-reported weight and height: Results of the Nutrinet-Santé study. Journal of Medical Internet Research. 2013;15(8):e152. doi: 10.2196/jmir.2575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Touvier M, Méjean C, Kesse-Guyot E, Pollet C, Malon A, Castetbon K, Hercberg S. Comparison between web-based and paper versions of a self-administered anthropometric questionnaire. European Journal of Epidemiology. 2010;25(5):287–296. doi: 10.1007/s10654-010-9433-9. [DOI] [PubMed] [Google Scholar]
- 42.Dascher, B. E. (2012). A gender-based approach to oral health changes across the lifespan. Anthropology Senior Theses, Paper 131. Philadelphia: University of Pennsylvania.
- 43.Lee KH, Xu H, Wu B. Gender differences in quality of life among community-dwelling older adults in low- and middle-income countries: Results from the Study on global AGEing and adult health (SAGE) BMC Public Health. 2020;20(1):114. doi: 10.1186/s12889-020-8212-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Samiei Siboni F, Alimoradi Z, Atashi V, Alipour M, Khatooni M. Quality of life in different chronic diseases and its related factors. International Journal of Preventive Medicine. 2019;10:65. doi: 10.4103/ijpvm.IJPVM_429_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Megari K. Quality of life in chronic disease patients. Health Psychology Research. 2013;1(3):e27. doi: 10.4081/hpr.2013.e27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Al Habashneh R, Khader YS, Salameh S. Use of the Arabic version of Oral Health Impact Profile-14 to evaluate the impact of periodontal disease on oral health-related quality of life among Jordanian adults. Journal of Oral Science. 2012;54(1):113–120. doi: 10.2334/josnusd.54.113. [DOI] [PubMed] [Google Scholar]
- 47.Brennan DS, Spencer AJ, Roberts-Thomson KF. Quality of life and disability weights associated with periodontal disease. Journal of Dental Research. 2007;86(8):713–717. doi: 10.1177/154405910708600805. [DOI] [PubMed] [Google Scholar]
- 48.Bourdiol P, Hennequin M, Peyron MA, Woda A. Masticatory adaptation to occlusal changes. Frontiers in Physiology. 2020;11:263. doi: 10.3389/fphys.2020.00263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Budtz-Jorgensen E, Chung JP, Rapin CH. Nutrition and oral health. Best Practice & Research Clinical Gastroenterology. 2001;15(6):885–896. doi: 10.1053/bega.2001.0247. [DOI] [PubMed] [Google Scholar]
- 50.Darnaud C, Thomas F, Danchin N, Boutouyrie P, Bouchard P. Masticatory capacity and mortality: The Preventive and Clinical Investigation Center (IPC) Cohort Study. Journal of Dental Research. 2020;99(2):152–158. doi: 10.1177/0022034519889021. [DOI] [PubMed] [Google Scholar]
- 51.Bouchard P, Carra MC, Boillot A, Mora F, Range H. Risk factors in periodontology: A conceptual framework. Journal of Clinical Periodontology. 2017;44(2):125–131. doi: 10.1111/jcpe.12650. [DOI] [PubMed] [Google Scholar]
- 52.Chapple IL, Bouchard P, Cagetti MG, Campus G, Carra MC, Cocco F, Nibali L, Hujoel P, Laine ML, Lingstrom P, Manton DJ, Montero E, Pitts N, Range H, Schlueter N, Teughels W, Twetman S, Van Loveren C, Van der Weijden F, Vieira AR, Schulte AG. Interaction of lifestyle, behaviour or systemic diseases with dental caries and periodontal diseases: Consensus report of group 2 of the joint EFP/ORCA workshop on the boundaries between caries and periodontal diseases. Journal of Clinical Periodontology. 2017;44(Suppl 18):S39–S51. doi: 10.1111/jcpe.12685. [DOI] [PubMed] [Google Scholar]
- 53.Eke PI, Dye BA, Wei L, Thornton-Evans GO, Genco RJ, CDC Periodontal Disease Surveillance workgroup: James Beck, G. D. R. P Prevalence of periodontitis in adults in the United States: 2009 and 2010. Journal of Dental Research. 2012;91(10):914–920. doi: 10.1177/0022034512457373. [DOI] [PubMed] [Google Scholar]
- 54.Hajishengallis G, Darveau RP, Curtis MA. The keystone-pathogen hypothesis. Nature Reviews Microbiology. 2012;10(10):717–725. doi: 10.1038/nrmicro2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Blicher B, Joshipura K, Eke P. Validation of self-reported periodontal disease: A systematic review. Journal of Dental Research. 2005;84(10):881–890. doi: 10.1177/154405910508401003. [DOI] [PubMed] [Google Scholar]
- 56.Abbood HM, Hinz J, Cherukara G, Macfarlane TV. Validity of self-reported periodontal disease: A systematic review and meta-analysis. Journal of Periodontology. 2016;87(12):1474–1483. doi: 10.1902/jop.2016.160196. [DOI] [PubMed] [Google Scholar]
- 57.Genco RJ, Borgnakke WS. Risk factors for periodontal disease. Periodontology 2000. 2013;62(1):59–94. doi: 10.1111/j.1600-0757.2012.00457.x. [DOI] [PubMed] [Google Scholar]
- 58.Persoon IF, Volgenant CMC, van der Veen MH, Opdam NJM, Manton DJ, Bruers JJM. Impact of the Coronavirus on providing oral health care in the Netherlands. International Dental Journal. 2021;S0020–6539(21):00228–238. doi: 10.1016/j.identj.2021.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Abedi N. Psychological effects of the COVID-19 pandemic on dentistry: A systematic review study. Journal of Education Health Promotion. 2021;10:311. doi: 10.4103/jehp.jehp_1637_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schmidt J, Waldova E, Balkova S, Suchanek J, Smucler R. Impact of COVID-19 on Czech dentistry: A nationwide cross-sectional preliminary study among dentists in the Czech Republic. International Journal of Environmental Research and Public Health. 2021;18(17):9121. doi: 10.3390/ijerph18179121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Group, C. O. C The COVID-19 pandemic and its global effects on dental practice. An International survey. J Dent. 2021;114:103749. doi: 10.1016/j.jdent.2021.103749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sanders AE, Slade GD, Fitzsimmons TR, Bartold PM. Physical activity, inflammatory biomarkers in gingival crevicular fluid and periodontitis. Journal of Clinical Periodontology. 2009;36(5):388–395. doi: 10.1111/j.1600-051X.2009.01394.x. [DOI] [PubMed] [Google Scholar]
- 63.Steele JG, Sanders AE, Slade GD, Allen PF, Lahti S, Nuttall N, Spencer AJ. How do age and tooth loss affect oral health impacts and quality of life? A study comparing two national samples. Community Dentistry and Oral Epidemiology. 2004;32(2):107–114. doi: 10.1111/j.0301-5661.2004.00131.x. [DOI] [PubMed] [Google Scholar]
- 64.Batista MJ, Lawrence HP, de Sousa Mda L. Impact of tooth loss related to number and position on oral health quality of life among adults. Health and Quality of Life Outcomes. 2014;12:165. doi: 10.1186/s12955-014-0165-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gerritsen AE, Allen PF, Witter DJ, Bronkhorst EM, Creugers NH. Tooth loss and oral health-related quality of life: A systematic review and meta-analysis. Health and Quality of Life Outcomes. 2010;8:126. doi: 10.1186/1477-7525-8-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gokturk O, Yarkac FU. Comparison of two measures to determine the oral health-related quality of life in elders with periodontal disease. Community Dental Health. 2019;36(2):143–149. doi: 10.1922/CDH_4387Gokturk07. [DOI] [PubMed] [Google Scholar]
- 67.Skaar DD, Hardie NA. Demographic factors associated with dental utilization among community dwelling elderly in the United States, 1997. Journal of Public Health Dentistry. 2006;66(1):67–71. doi: 10.1111/j.1752-7325.2006.tb02554.x. [DOI] [PubMed] [Google Scholar]
- 68.Skaar DD, O'Connor H. Dental service trends for older US adults, 1998–2006. Special Care in Dentistry. 2012;32(2):42–48. doi: 10.1111/j.1754-4505.2012.00239.x. [DOI] [PubMed] [Google Scholar]
- 69.Ikebe K, Hazeyama T, Enoki K, Murai S, Okada T, Kagawa R, Matsuda K, Maeda Y. Comparison of GOHAI and OHIP-14 measures in relation to objective values of oral function in elderly Japanese. Community Dentistry and Oral Epidemiology. 2012;40(5):406–414. doi: 10.1111/j.1600-0528.2012.00683.x. [DOI] [PubMed] [Google Scholar]
- 70.Rodakowska E, Mierzyńska K, Bagińska J, Jamiołkowski J. Quality of life measured by OHIP-14 and GOHAI in elderly people from Bialystok, north-east Poland. BMC Oral Health. 2014;14:106. doi: 10.1186/1472-6831-14-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Miksch A, Hermann K, Rölz A, Joos S, Szecsenyi J, Ose D, Rosemann T. Additional impact of concomitant hypertension and osteoarthritis on quality of life among patients with type 2 diabetes in primary care in Germany - a cross-sectional survey. Health and Quality of Life Outcomes. 2009;7:19. doi: 10.1186/1477-7525-7-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shay B, Ben Ami O, Levy Ianculovici D, Zini A, Ianculovici C, Almoznino G. Oral health-related quality of life in patients with disorders of nutrition. Journal of Oral Rehabilitation. 2019;46(4):355–368. doi: 10.1111/joor.12754. [DOI] [PubMed] [Google Scholar]
- 73.Andreeva VA, Egnell M, Galan P, Feron G, Hercberg S, Julia C. Association of the dietary index underpinning the Nutri-Score label with oral health: Preliminary evidence from a large, population-based sample. Nutrients. 2019;11(9):1998. doi: 10.3390/nu11091998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Andreeva VA, Salanave B, Castetbon K, Deschamps V, Vernay M, Kesse-Guyot E, Hercberg S. Comparison of the sociodemographic characteristics of the large NutriNet-Sante e-cohort with French Census data: The issue of volunteer bias revisited. Journal of Epidemiology and Community Health. 2015;69(9):893–898. doi: 10.1136/jech-2014-205263. [DOI] [PubMed] [Google Scholar]
- 75.Zong G, Scott AE, Griffiths HR, Zock PL, Dietrich T, Newson RS. Serum alpha-tocopherol has a nonlinear inverse association with periodontitis among US adults. Journal of Nutrition. 2015;145(5):893–899. doi: 10.3945/jn.114.203703. [DOI] [PubMed] [Google Scholar]
- 76.Alt KW, Rossbach A. Nothing in nature is as consistent as change. Frontiers of Oral Biology. 2009;13:190–196. doi: 10.1159/000242416. [DOI] [PubMed] [Google Scholar]
- 77.Bourgeois D, Bouchard P, Mattout C. Epidemiology of periodontal status in dentate adults in France, 2002–2003. Journal of Periodontal Research. 2007;42(3):219–227. doi: 10.1111/j.1600-0765.2006.00936.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.