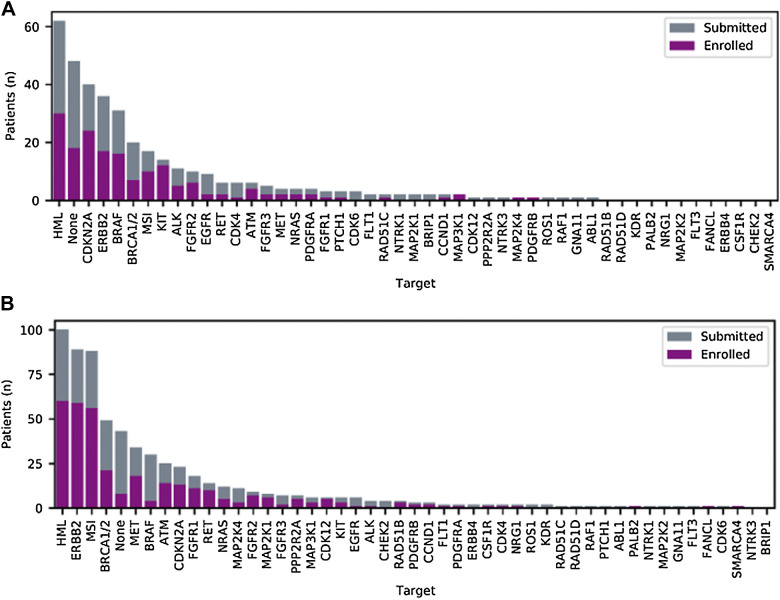
Figure 2.
Molecular targets used for submission/enrollment. Representation of all genes harboring somatic alterations that were reviewed by the study team and enrolled in the trial of rare cancer (A) and nonrare cancer (B) patients. For enrolled patients, the target depicted was matched for treatment. None indicates that only the RAF/RAS wild-type status could confer anti-EGFR treatment (panitumumab) or that there was no actionable target present.