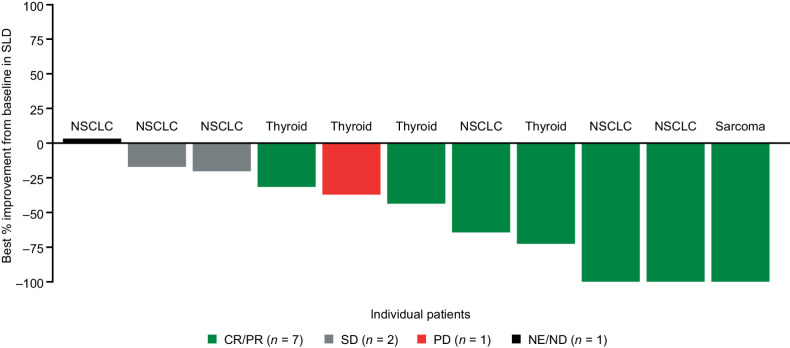
Figure 3.
Best intracranial responses to entrectinib in patients with NTRK fusion-positive solid tumors and measurable CNS metastases (BICR assessed) at baseline. Data cut-off August 31, 2020. Response assessed by BICR. Patients with new CNS lesions or unequivocal progression of nontarget lesions had overall response classified as PD, even if the SLD of all lesions was reduced. ND, not determined; SD, stable disease.