Abstract
Purpose:
Primary analysis of VISION showed tepotinib had durable clinical activity in patients with MET exon 14 (METex14) skipping non–small cell lung cancer (NSCLC). We present updated outcomes for clinically relevant subgroups.
Patients and Methods:
This phase II, open-label, multi-cohort study of 500 mg (450 mg active moiety) tepotinib in patients with METex14 skipping NSCLC assessed efficacy and safety in predefined subgroups according to age, prior therapies (chemotherapy and immune checkpoint inhibitors), and brain metastases. An ad hoc retrospective analysis using Response Assessment in Neuro-Oncology Brain Metastases (RANO-BM) criteria assessed intracranial activity.
Results:
152 patients were evaluable for efficacy (median age: 73.1). Overall, objective response rate (ORR) was 44.7% [95% confidence interval (CI): 36.7–53.0]. Patients aged <75 (n = 84) and ≥75 (n = 68) had ORRs of 48.8% (95% CI: 37.7–60.0) and 39.7% (95% CI: 28.0–52.3), respectively. Treatment-naïve (n = 69) versus previously treated (n = 83) patients showed consistent efficacy [ORR (95% CI): 44.9% (32.9–57.4) vs. 44.6% (33.7–55.9); median duration of response (95% CI): 10.8 (6.9–not estimable) vs. 11.1 (9.5–18.5) months]. Of 15 patients analyzed by RANO-BM (12 received prior radiotherapy), 13 achieved intracranial disease control; 5 of 7 patients with measurable brain metastases had partial intracranial responses. Of 255 patients evaluable for safety, 64 (25.1%) experienced grade ≥3 treatment-related adverse events (TRAE), leading to discontinuation in 27 patients (10.6%). Rates of adverse events (AE) were broadly consistent irrespective of prior therapies.
Conclusions:
Tepotinib showed meaningful activity across subgroups by age, prior therapies, and brain metastases, with a manageable safety profile and few treatment discontinuations.
See related commentary by Rosner and Spira, p. 1055
Translational Relevance.
Tepotinib, a highly selective and potent MET inhibitor, has demonstrated durable clinical activity in MET exon 14 (METex14) skipping non–small cell lung cancer (NSCLC). To better inform clinical practice, we investigated outcomes with tepotinib in patient subgroups of particular relevance from the VISION study, including advanced age, prior therapies, and brain metastases. Additionally, we performed the first assessment of intracranial activity of tepotinib, in an ad hoc retrospective analysis using Response Assessment in Neuro-Oncology Brain Metastases (RANO-BM) criteria. Meaningful clinical activity was observed across age groups, including in patients ≥80 years, which is reassuring given the advanced age of patients with METex14 skipping NSCLC. Tepotinib was also effective regardless of whether prior therapies were received, alleviating potential concerns regarding sequencing of tepotinib. Intracranial disease control was achieved in 13 of 15 evaluable patients, indicating that tepotinib may be beneficial for intracranial disease control.
Introduction
MET exon 14 (METex14) skipping is an oncogenic driver occurring in 3–4% of non–small cell lung cancer (NSCLC) cases, and defines a patient population that benefit from targeted MET inhibitors (1–3). Concomitant driver alterations are uncommon and, compared with patients with NSCLC harboring other oncogenic drivers, this patient population is typically older, more evenly distributed by gender, has a higher proportion of patients with smoking history, and a higher proportion of tumors expressing high levels of programmed death-ligand 1 (PD-L1; refs. 1, 4, 5). Up to approximately 40% of METex14 skipping NSCLC cases also involve brain lesions, presenting a high unmet medical need (6–9). Until the recent approval of MET inhibitors, treatment patterns for METex14 skipping NSCLC have been heterogeneous, with treatments including immune checkpoint inhibitors (ICI) and chemotherapy, alone or in combination, and off-label use of multi-kinase inhibitors such as crizotinib (10, 11).
Tepotinib is an oral, once daily, highly selective MET tyrosine kinase inhibitor (TKI), with established clinical activity in patients with MET-driven tumors, and is blood–brain barrier penetrant (25% of tepotinib in plasma is able to cross into the brain; refs. 12–14). In the first reported efficacy analysis from VISION, comprising 99 patients with ≥9 months' follow-up, tepotinib had an objective response rate (ORR) of 46.5% across lines of therapy according to independent review committee (IRC; ref. 15). Based on results from VISION, tepotinib was the first agent approved for advanced NSCLC with METex14 skipping, in Japan in March 2020, and was subsequently approved in the United States, Brazil, Canada, Taiwan, Republic of Korea, Singapore, Great Britain, and Switzerland in 2021. Guidelines now include tepotinib for advanced NSCLC harboring METex14 skipping, regardless of lines of prior therapy (16–18).
Given the recent development of MET-targeted therapies, understanding outcomes in different patient populations will be important to inform clinical practice. We investigated the most recent data from the VISION study, now comprising a larger population with all patients enrolled in cohort A, in which meaningful subgroup analyses can be conducted, to address knowledge gaps in patient subgroups of particular relevance to METex14 skipping NSCLC, including advanced age, prior therapies, and brain metastases.
Patients and Methods
Study design and objectives
VISION (NCT02864992) is a phase II, single-arm, multi-cohort, open-label, trial in more than 130 sites across 14 countries to assess antitumor activity and tolerability of tepotinib in advanced NSCLC harboring METex14 skipping in cohorts A and C, as described previously (15). Cohort A serves as the primary analysis set, and cohort C will serve as a confirmatory cohort for cohort A. At the time of analysis, enrollment into cohort A was complete, and was ongoing for cohort C.
All patients provided written informed consent and the study was done in accordance with the Declaration of Helsinki, International Conference on Harmonization Good Clinical Practice, local laws, and applicable regulatory requirements. The study was approved by the institutional review board or independent ethics committee of each center.
Patients and treatment
Patients were aged ≥18 years with histologically or cytologically confirmed advanced (locally advanced or metastatic) NSCLC with METex14 skipping detected by liquid and/or tissue biopsy, measurable disease, and Eastern Cooperative Oncology Group performance status 0/1. Patients with tumors harboring EGFR mutations or ALK rearrangements, or who had received more than two lines of prior therapy, were not eligible. Prior immunotherapy was allowed but prior MET inhibitors were not.
Patients with asymptomatic brain metastases were eligible. Patients were excluded if they had symptomatic brain metastases, were neurologically unstable, required an increase in steroid dose within 2 weeks, received prior stereotactic radiosurgery/Gamma Knife within 2 weeks, received other prior treatment for brain metastases within 4 weeks, or had leptomeningeal disease.
Eligible patients received tepotinib 500 mg (450 mg active moiety) orally once daily until disease progression, intolerable toxicity, or withdrawal of consent.
Study endpoints and assessments
The primary endpoint was objective response, determined by IRC per Response Evaluation Criteria in Solid Tumors (RECIST) v1.1. Secondary endpoints were investigator-assessed objective response, duration of response (DOR), progression-free survival (PFS), overall survival (OS), and safety. Tumor assessments were conducted every 6 weeks within the first 9 months of treatment (and every 12 weeks thereafter) by computed tomography or magnetic resonance imaging (MRI) of the chest, abdomen, and pelvis. Prior to a protocol amendment released in January 2020, at which time cohort A had completed enrollment, brain imaging at baseline was not required (15).
Safety was evaluated using clinical laboratory tests and physical examination. Adverse events (AE) were assessed using the NCI Common Terminology Criteria for Adverse Events v4.03. An independent panel conducted a retrospective review of interstitial lung disease (ILD)-like events identified by a composite term search.
Prior therapies, received before study entry, were classified using the World Health Organization Drug Dictionary. Outcomes were not monitored as part of a clinical study and, as such, best responses were determined by the investigator's clinical judgement; no scheduled assessments and no standard criteria were specified.
An ad hoc retrospective analysis using Response Assessment in Neuro-Oncology Brain Metastases (RANO-BM) criteria was conducted to assess intracranial activity in patients with brain lesions (identified by IRC) and ≥1 evaluable post-baseline tumor assessment. Further information on response assessment in this analysis is provided in the Supplemental Methods.
Statistical analysis
Patients enrolled in cohort A were assessed for efficacy and safety. Due to ongoing enrollment, and therefore limited follow-up at the time of analysis, patients enrolled in cohort C are included in safety analyses only. No formal statistical comparisons were conducted; data were analyzed in a descriptive manner. Subgroup analyses according to baseline characteristics, including age, prior treatments, and presence of baseline brain metastases were preplanned. Kaplan–Meier methods were used to analyze the DOR, PFS, and OS.
Data availability statement
Any requests for data by qualified scientific and medical researchers for legitimate research purposes will be subject to the healthcare business of Merck KGaA, Darmstadt, Germany, Data Sharing Policy. All requests should be submitted in writing to the healthcare business of Merck KGaA, Darmstadt, Germany, data sharing portal (https://www.emdgroup.com/en/research/our-approach-to-research-and-development/healthcare/clinical-trials.html).
Results
Patients
As of July 1, 2020, 152 patients in cohort A were analyzed as the efficacy population. In this population, median duration of treatment was 7.0 months (range <0.1–43.3), and median follow-up duration was 16.4 months (range 0.3–43.3); 28 patients (18.4%) remained on treatment at time of analysis. Median age was 73.1 years (range 41–94), 52.0% were male and 52.0% had smoking history. Baseline demographics in subgroups are shown in Table 1. Two hundred and fifty-five patients were enrolled across cohorts A and C, and comprised the safety population.
Table 1.
Baseline patient characteristics (efficacy population).
| Overall cohort A (N = 152) | Treatment-naïve (n = 69) | Previously treated (n = 83) | <75 years (n = 84) | ≥75 years (n = 68) | ||
|---|---|---|---|---|---|---|
| Median age, years (range) | 73.1 (41–94) | 74.0 (56–94) | 72.6 (41–88) | 68.4 (41–75) | 80.0 (75–94) | |
| Sex, n (%) | Male | 79 (52.0) | 36 (52.2) | 43 (51.8) | 44 (52.4) | 35 (51.5) |
| Female | 73 (48.0) | 33 (47.8) | 40 (48.2) | 40 (47.6) | 33 (48.5) | |
| Race, n (%)a | Asian | 38 (25.0) | 12 (17.4) | 26 (31.3) | 25 (29.8) | 13 (19.1) |
| Caucasian/White | 108 (71.1) | 56 (81.2) | 52 (62.7) | 56 (66.7) | 52 (76.5) | |
| Smoking history, n (%)b | Yes | 79 (52.0) | 43 (62.3) | 36 (43.4) | 51 (60.7) | 28 (41.2) |
| No | 65 (42.8) | 26 (37.7) | 39 (47.0) | 27 (32.1) | 38 (55.9) | |
| ECOG PS, n (%) | 0 | 41 (27.0) | 25 (36.2) | 16 (19.3) | 25 (29.8) | 16 (23.5) |
| 1 | 111 (73.0) | 44 (63.8) | 67 (80.7) | 59 (70.2) | 52 (76.5) | |
| Histologic subtype, n (%)c | Adenocarcinoma | 131 (86.2) | 58 (84.1) | 73 (88.0) | 75 (89.3) | 56 (82.4) |
| Squamous | 15 (9.9) | 6 (8.7) | 9 (10.8) | 8 (9.5) | 7 (10.3) | |
| Sarcomatoid | 3 (2.0) | 3 (4.3) | 0 | 0 | 3 (4.4) | |
| Lines of prior therapy, n (%)d | 0 | 69 (45.4) | 69 (100) | 0 | 37 (44.0) | 32 (47.1) |
| 1 | 49 (32.2) | 0 | 49 (59.0) | 29 (34.5) | 20 (29.4) | |
| 2+ | 34 (22.4) | 0 | 34 (41.0) | 18 (21.4) | 16 (23.5) | |
| Brain metastases at baseline, n (%)e | 23 (15.1) | 10 (14.5) | 13 (15.7) | 15 (17.9) | 8 (11.8) | |
| Identification of METex14 skippingf | Liquid biopsy | 99 (65.1) | 44 (63.8) | 55 (66.3) | 56 (66.7) | 43 (63.2) |
| Tissue biopsy | 88 (57.9) | 42 (60.9) | 46 (55.4) | 51 (60.7) | 37 (54.4) | |
Abbreviation: ECOG PS, Eastern Cooperative Oncology Group performance status.
aRace was unknown or missing in 4 patients, 1 patient was Black/African American, and 1 patient was ‘other’.
bSmoking history was missing in 8 patients.
cTwo patients had adenosquamous histology (1 treatment-naïve and 1 previously treated), and 1 patient (treatment-naïve) had NSCLC not otherwise specified.
dLines of prior therapy for advanced/metastatic disease.
eBrain metastases at baseline as identified per RECIST v1.1 criteria; a total of 81 patients had brain scans that were submitted to the imaging vendor for the study.
fPatients could have a positive test for METex14 skipping by both liquid and tissue biopsy (as shown in Supplementary Fig. S5).
Outcomes in the cohort A efficacy population
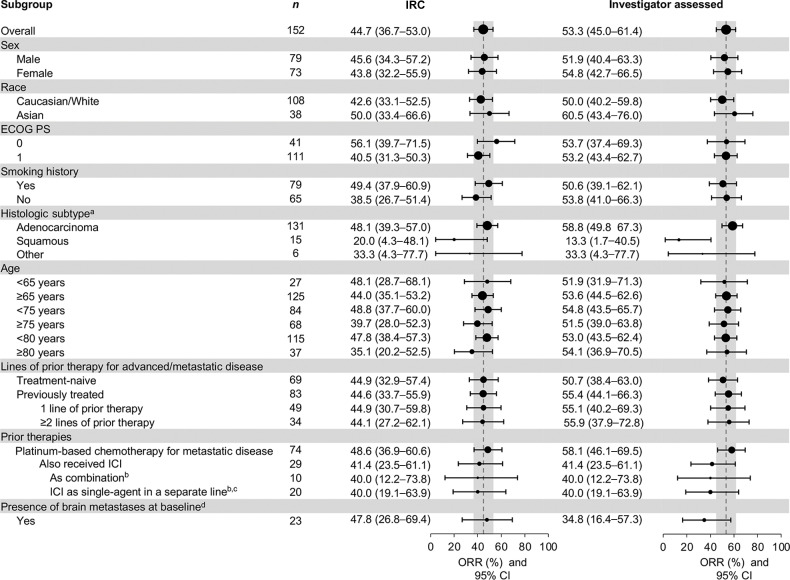
In the efficacy population, 68 of 152 patients achieved an objective response by IRC, for an ORR of 44.7% [95% confidence interval (CI): 36.7–53.0]. ORR in subgroups according to baseline characteristics is shown in Fig. 1. Responses were rapid, with 57 of 68 (83.8%) recorded at first (6 weeks) or second (12 weeks) tumor assessments (Fig. 2A and B). The median (m) DOR was 11.1 months (95% CI: 8.4–18.5), and mPFS was 8.9 months (95% CI: 8.2–11.2; Table 2; Supplementary Figs. S1 and S2). Investigator-assessed outcomes are shown in Supplementary Table S1 and Supplementary Figs. S3 and S4; ORR was 53.3% (95% CI: 45.0–61.4), with an mDOR of 12.5 months (95% CI: 9.7–18.3). Both ORR and PFS in patients enrolled by liquid or tissue biopsy were similar, shown in Supplementary Table S2, and proportions of patients enrolled based on each method are shown in Supplementary Fig. S5. There were 146 patients with ≥9 months' follow-up at the time of analysis, and outcomes in these patients can be found in Supplementary Table S3. At the time of analysis, 76 patients (50.0%) had died and mOS was 17.6 months (95% CI: 15.0–21.0; Supplementary Fig. S6).
Figure 1.
ORR in subgroups. aOf 15 patients with squamous cell histology; 9 (60.0%) had a smoking history and 6 (40.0%) were never smokers; 7 were from the United States (46.7%), 5 from Europe (33.3%), and 3 from Asia (20.0%). ‘Other’ histologies included sarcomatoid (n = 3), adenosquamous (n = 2), and NSCLC not otherwise specified (n = 1). bOne patient received ICI as monotherapy and in combination with platinum-based chemotherapy and, as such, is included in both subgroups. cPatients could have received first-line platinum-based chemotherapy followed by second-line single-agent ICI, or vice versa. dBrain metastases as identified by RECIST v1.1 criteria; systemic objective response per RECIST v1.1 criteria.
Figure 2.
Response to tepotinib according to line of therapy. A, The change in sum of longest diameters between baseline and best post-baseline assessment by independent review. Three treatment-naïve patients excluded due to baseline/on-treatment measurement not being available. Six patients (4 treatment-naïve and 2 previously treated) who had more than 30% tumor shrinkage of target lesions according to IRC had a BOR of PD because of the growth of new lesions or PD in non-target lesions. According to investigator assessment, these patients had BORs of PR (n = 5) and SD (n = 1), and they remained on treatment for between 4.1 and 17.7 months, with 2 patients ongoing at the time of analysis. Reasons for treatment discontinuation were PD (n = 2), AEs (n = 1), and protocol non-compliance (n = 1). B, Time on treatment, time to, and duration of response by investigator assessment. Prior therapies received by previously treated patients were platinum-based chemotherapy for metastatic disease (n = 74) and ICI (n = 29; in combination or as monotherapy in a separate line), or others (including other cytotoxic therapies, monoclonal antibodies, and small molecules). C, PFS with tepotinib, and corresponding time to progression, as evaluated by the investigator, with prior ICI. Responses and PFS with tepotinib assessed by investigator. Only patients for whom time to progression data with prior ICI was available (n = 28) are shown. Time to progression with prior ICI for the patient denoted with an asterisk (*) was reported as 0 months. NE, not evaluable.
Table 2.
Tepotinib efficacy according to IRC (efficacy population).
| Treatment-naïve (n = 69) | Previously treated (n = 83) | Overall (N = 152) | |
|---|---|---|---|
| BOR, n (%) | |||
| CR | 0 | 0 | 0 |
| PR | 31 (44.9) | 37 (44.6) | 68 (44.7) |
| SD | 16 (23.2) | 23 (27.7) | 39 (25.7) |
| PD | 13 (18.8) | 13 (15.7) | 26 (17.1) |
| NE | 9 (13.0) | 10 (12.0) | 19 (12.5) |
| ORR, % | 44.9 | 44.6 | 44.7 |
| (95% CI) | (32.9–57.4) | (33.7–55.9) | (36.7–53.0) |
| DCR, % | 68.1 | 72.3 | 70.4 |
| (95% CI) | (55.8–78.8) | (61.4–81.6) | (62.5–77.5) |
| Median DOR, months | 10.8 | 11.1 | 11.1 |
| (95% CI) | (6.9–ne) | (9.5–18.5) | (8.4–18.5) |
| Median PFS, months | 8.5 | 10.9 | 8.9 |
| (95% CI) | (6.8–11.3) | (8.2–12.7) | (8.2–11.2) |
Abbreviation: DCR, disease control rate.
Outcomes according to age
As METex14 skipping often occurs in patients with NSCLC with older age (19), analysis was performed according to age. ORR by IRC in patients <75 years was 48.8% (95% CI: 37.7–60.0), and 39.7% (95% CI: 28.0–52.3) in patients ≥75 years (Fig. 1). Patients ≥80 years (n = 37) had an ORR of 35.1% (95% CI: 20.2–52.5), disease control rate of 67.6% (95% CI: 50.2–82.0), mDOR of 10.1 months (95% CI: 5.6–18.5), and mPFS of 8.6 months (95% CI: 5.4–15.3). mDOR and mPFS in subgroups according to age are shown in Supplementary Figs. S1–S4.
Outcomes according to treatment sequencing
ORRs by IRC were comparable regardless of therapy line, with 44.9% (95% CI: 32.9–57.4) in treatment-naïve patients and 44.6% (95% CI: 33.7–55.9) in previously treated patients (Table 2 and Fig. 1). In treatment-naïve patients, mDOR was 10.8 months [95% CI: 6.9–not estimable (ne)] and mPFS was 8.5 months (95% CI: 6.8–11.3). In previously treated patients, mDOR was 11.1 months (95% CI: 9.5–18.5) and mPFS was 10.9 months (95% CI: 8.2–12.7). Upon further investigation of IRC assessments, 2 patients identified as responders in our analysis (1 treatment-naïve, 1 previously treated) could be considered non-responders based on additional information (see Supplemental Results), producing an ORR of 43.5% (95% CI: 31.6–56.0) in treatment-naïve patients, and 43.4% (95% CI: 32.5–54.7) in previously treated patients (20, 21).
Next, we evaluated patient subgroups by types of prior therapies for metastatic disease. Common prior therapies were platinum-based chemotherapy for metastatic disease (n = 74) and ICI (n = 29; most commonly pembrolizumab and nivolumab); 10 patients received these in combination. ORR was consistent in patients who received prior chemotherapy for metastatic disease or ICI (in combination or as monotherapy in a separate line), with 95% CIs largely overlapping across these subgroups (Fig. 1). In patients who received platinum-based chemotherapy for metastatic disease, mDOR was 12.4 months (95% CI: 9.5–18.5) and mPFS was 11.0 months (95% CI: 8.2–13.7; Supplementary Figs. S1 and S2). In patients who also received prior ICI (n = 29; in combination or as monotherapy in a separate line), mDOR was 9.5 months (95% CI: 6.9–ne) and mPFS was 10.9 months (95% CI: 3.0–12.7). There was no apparent association between PFS with tepotinib and the corresponding time to progression with their prior ICI regimen, as evaluated by the investigator (Fig. 2C). Median time to progression with prior ICI was 4.5 months (range 0–36).
Of 83 previously treated patients, clinical benefit data (as assessed by the physician), was available for 75 patients. Twenty-one of 75 patients (28%) had a response to their most recent anticancer therapy with a median time to progression of 4.0 months (range 0–36; Supplementary Table S4).
Of 124 patients in the efficacy population who discontinued tepotinib [77 due to progressive disease (PD), 26 due to AEs, 12 due to death, 9 for other reasons], 47 received subsequent treatment (Supplementary Fig. S7), the majority of whom were <75 years old (n = 36; Supplementary Table S5). At the time of analysis, 31 patients had received one line of subsequent therapy, 14 patients received two subsequent lines, and 1 patient each received three and five subsequent lines. Of patients for whom response data were available (n = 22), tumor responses were observed in 22.7% of patients, across all subsequent therapies. The most common types of subsequent therapies were cytotoxic therapy and ICI. Twelve patients received crizotinib, 3 received capmatinib, and 2 received cabozantinib (Supplementary Table S6). Of the 12 patients who received subsequent crizotinib, most (n = 9) received crizotinib immediately after tepotinib, and 3 patients received other therapies between tepotinib and crizotinib (2 patients received ICI/chemotherapy regimens, and 1 patient received immunotherapy as monotherapy). Responses with crizotinib were not available; however, time on treatment ranged from <1–3 months. Outcomes with capmatinib or cabozantinib were not available.
Intracranial activity of tepotinib
Twenty-three patients with baseline brain metastases (per RECIST v1.1) were enrolled, 17 of whom received prior radiotherapy. Systemic ORR was 47.8% (95% CI: 26.8–69.4; Fig. 1), mDOR was 9.5 months (95% CI: 5.5–ne), and mPFS was 9.5 months (95% CI: 5.7–11.2). New brain lesions were detected by the investigator in 6 of 152 patients, 4 of whom had baseline brain lesions.
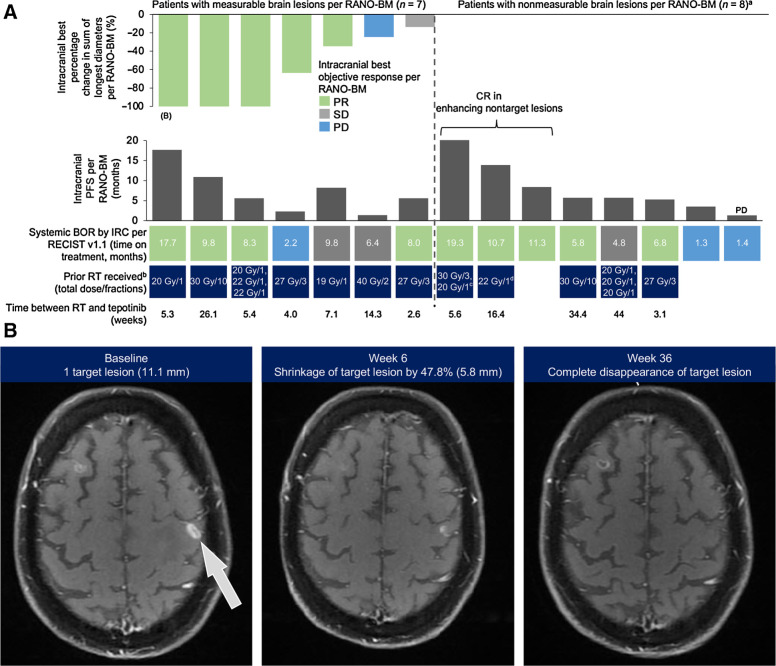
A total of 15 patients had brain lesions that were considered eligible by independent reviewers for intracranial response assessment, according to RANO-BM criteria. Of these, 12 received prior radiotherapy with a median time between radiotherapy and tepotinib of 6.4 weeks (range 2.6–44). Intracranial disease control was achieved in 13 patients. Seven patients had measurable brain lesions that were evaluable for response; all had received prior radiotherapy. Intracranial best objective responses (BOR) were partial response (PR; n = 5, including 3 with complete disappearance of target lesions), stable disease (SD; n = 1), and PD (n = 1). Of 8 patients with non-measurable brain lesions only [enhancing non-target lesions (NTL)], 7 achieved intracranial disease control, including 3 with complete response (CR) in the enhancing NTLs, and one had an intracranial BOR of PD (Fig. 3).
Figure 3.
Intracranial responses to tepotinib assessed by IRC according to RANO-BM criteria. A, Waterfall plot depicting intracranial best change in sum of longest diameters (patients with measurable lesions only), and intracranial PFS. B, MRI images showing response to tepotinib in an intracranial target lesion per RANO-BM criteria; this patient is indicated in A. a7 of 8 patients with non-measurable brain lesions achieved intracranial disease control; 1 patient had intracranial BOR of PD (indicated on graph). bRT for brain lesions. c20 Gy in 1 fraction was reported during the same time period as 30 Gy in 3 fractions. dGamma Knife was also received 31.4 weeks prior to the start of tepotinib treatment. Gy, Gray (unit); RT, radiotherapy.
Safety
All patients enrolled across VISION cohorts A and C, as of July 1, 2020 (n = 255), were assessed for safety, irrespective of follow-up duration. Median treatment duration was 5.1 months (range <0.1–43.3), with 101 patients still on treatment. AEs of any cause were reported in 96.5% of patients. No AEs due to COVID-19 were reported at the time of analysis. Treatment-related AEs (TRAE) were reported in 86.3% of patients, and 24.3% experienced TRAEs of grades 3 to 4 (Table 3). Serious TRAEs occurred in 12.2% of patients (Supplementary Table S7). Three TRAEs were fatal: acute respiratory failure secondary to ILD, severe worsening of dyspnea, and acute hepatic failure. Death due to hepatic failure occurred after the patient had withdrawn consent to continue participating in the study.
Table 3.
TRAEs occurring in ≥5% of all patients (any grade, safety population).
| All METex14 skipping NSCLC cohorts A + C (N = 255) | ||
|---|---|---|
| Category, n (%) | All grades | Grade 3–4 |
| Any AEa | 220 (86.3) | 62 (24.3) |
| Peripheral edema | 138 (54.1) | 19 (7.5) |
| Nausea | 51 (20.0) | 1 (0.4) |
| Diarrhea | 50 (19.6) | 1 (0.4) |
| Blood creatinine increased | 45 (17.6) | 1 (0.4) |
| Hypoalbuminemia | 37 (14.5) | 6 (2.4) |
| Alanine aminotransferase increased | 22 (8.6) | 5 (2.0) |
| Decreased appetite | 21 (8.2) | 1 (0.4) |
| Amylase increased | 19 (7.5) | 5 (2.0) |
| Fatigue | 18 (7.1) | 1 (0.4) |
| Alopecia | 18 (7.1) | 0 |
| Lipase increased | 17 (6.7) | 7 (2.7) |
| Pleural effusion | 16 (6.3) | 8 (3.1) |
| Edema | 16 (6.3) | 0 |
| Aspartate aminotransferase increased | 15 (5.9) | 3 (1.2) |
| Constipation | 15 (5.9) | 0 |
| Asthenia | 14 (5.5) | 1 (0.4) |
| Vomiting | 14 (5.5) | 1 (0.4) |
| Upper abdominal pain | 14 (5.5) | 0 |
aTwo patients had TRAEs that were fatal during the study; 1 patient had acute respiratory failure secondary to ILD, and 1 patient had severe worsening of dyspnea. Another patient had fatal acute hepatic failure after withdrawing consent to continue participating in the study.
There were six reports of ILD-like events, as reviewed by the independent panel, including one fatal event described above. The events confirmed to be ILD-like, as initially reported by the investigator, were pneumonitis (n = 3), ILD (n = 2), and acute respiratory failure (n = 1), with time to onset ranging from 21 to 295 days. Treatment was permanently discontinued in 3 patients and was interrupted in 3 patients.
Peripheral edema was the most common TRAE, with 54.1% of patients experiencing any grade and 7.5% experiencing grade ≥3 events (Table 3). Dose reductions (14.1%) and interruptions (16.1%) were common, but permanent discontinuation due to peripheral edema was rare (3.5%; Supplementary Table S8). TRAEs led to dose reductions in 27.8% of patients, treatment interruptions in 35.3%, and permanent discontinuation in 10.6%. Other TRAEs leading to permanent discontinuation in more than 1% of patients were pleural effusion (1.2%), dyspnea (1.2%), and pneumonitis (1.2%). In the efficacy population (n = 152), 56 patients had dose reductions due to AEs of any cause, with 35 occurring in the first 5 months of treatment. Patients with dose reductions remained on treatment for prolonged periods, with 14 patients still on treatment at the time of analysis (Supplementary Fig. S8).
Incidence of TRAEs and treatment modifications in subgroups are shown in Supplementary Table S9. In patients younger or older than 75 years, TRAEs led to dose reductions in 23.3% and 33.9% of patients, treatment interruptions in 28.8% and 44.0%, and permanent discontinuation in 7.5% and 14.7%, respectively. In patients who received prior ICI, incidence of liver enzyme increases (any grade: alanine aminotransferase, 9.1%; aspartate aminotransferase, 7.6%) were consistent with the overall study population (8.6%; 5.9%). There were no reports of pneumonitis in patients who received prior ICI, and 1 patient had ILD (grades 1–2, confirmed by the independent panel). In this instance, ILD was managed with treatment interruptions, dose reductions, and steroid treatment; this patient received tepotinib treatment for a total of 11 months; further details are provided in Supplemental Results.
Discussion
Here, we report robust and durable clinical activity of tepotinib in patients with advanced NSCLC with METex14 skipping, confirming previous analysis from VISION in a larger patient population with an average follow-up of more than 1 year. In particular, the primary endpoint of ORR was consistent between treatment-naïve (44.9%) and previously treated (44.6%) patients, supporting the oncogenic driver concept for METex14 skipping.
Given the advanced age of patients with METex14 skipping NSCLC, it is reassuring that meaningful clinical activity was observed across age groups, including in patients ≥80 years. In studies of capmatinib or crizotinib, where the median ages were 71 and 72 respectively, clinical activity was observed in patients above and below 65 years (22, 23). Older patients are often more challenging to treat due to high concomitant medication use as a result of comorbidities and, as such, treatment effects on symptoms and functioning are particularly important. Tepotinib had a manageable safety profile in patients ≥75 years, with mostly mild–to–moderate AEs. In our study, most patients who received subsequent therapies were less than 75 years, highlighting the importance of prioritizing effective TKI therapies early in the treatment sequence for elderly patients.
As selective MET TKIs have only recently received regulatory approval, few data are available to guide the sequencing of MET TKIs with other standard treatment options, including chemotherapy and ICI. Understanding the limits imposed by the retrospective nature of this endeavor, we sought to address questions regarding the impact of prior therapies on the efficacy and safety of tepotinib, with a particular focus on ICI. Tepotinib was consistently effective across all lines of therapy. For those patients who received prior therapies, tepotinib was effective regardless of the type of prior therapy administered. This consistency mirrors our experience with other oncogenic drivers and their matched therapies, and is an assurance for those patients in whom molecular testing is, for many practical reasons, delayed (16). Additionally, tepotinib-related AEs did not vary by line of therapy, or by type of prior therapy, including prior ICI. This observation is of particular importance in patients with METex14 skipping NSCLC, whose tumors often bear high PD-L1 expression, and who will not infrequently receive ICI-based therapies while awaiting next-generation sequencing results (5). Prior reports have shown modest efficacy of ICI in METex14 skipping NSCLC (5, 24), consistent with observations for patients in VISION who received ICI prior to tepotinib; however, efficacy data for therapies received prior to study entry are limited by the absence of independent assessment, scheduled assessments, and standardized criteria. The safety profile was similar in patients who received ICI before tepotinib, suggesting that this sequence can be used without significantly increased concerns. Finally, we note that a significant subset of patients (51%), mostly ≥75 years of age, failed to receive subsequent treatments. This is reflective of the clinical observation that elderly patients have limited opportunities for multiple lines of systemic therapy and highlights the importance of providing the most effective treatment upfront. Efficacy of subsequent therapies was comparable with the efficacy of non-MET targeted therapies received prior to tepotinib. With multiple anti-MET TKIs available, it is of great interest to understand whether sequential use of TKIs can be beneficial. Capmatinib after crizotinib demonstrated only moderate activity, including in patients who discontinued crizotinib due to intolerance (25). In our cohort, crizotinib after tepotinib produced limited benefit (1–3 months treatment duration on crizotinib). Future studies are warranted in this space. Taken together, our data alleviate potential concerns regarding the sequencing of tepotinib to later lines, while bolstering the rationale to provide it early in the disease course, particularly for elderly patients.
While VISION was not designed to assess the intracranial activity of tepotinib in patients with METex14 skipping NSCLC, an ad hoc independent analysis using RANO-BM criteria demonstrated intracranial activity, with the caveat that most patients had received prior radiotherapy. Nonetheless, these data are in line with reported case studies (26, 27), including the report of a patient in whom tepotinib achieved high cerebrospinal fluid concentrations and demonstrated efficacy against leptomeningeal metastasis (28). Post hoc intracranial response analysis of capmatinib using RECIST v1.1 criteria showed that of 13 patients with brain metastases (6 of whom received prior radiotherapy), 7 had intracranial responses (22), and preliminary data also indicate savolitinib has antitumor activity in brain metastases (29). Therefore, systemic therapy using a MET inhibitor with intracranial activity may be particularly beneficial for both systemic and intracranial disease control, in line with recent guidelines recommending brain-penetrant TKIs for NSCLC with driver mutations and brain metastases (30).
Tepotinib's known safety profile was confirmed in this updated analysis, comprising the largest safety dataset available for a MET TKI in METex14 skipping NSCLC. The most common TRAE was peripheral edema, and there was a low frequency of treatment discontinuation, indicating TRAEs were manageable. Peripheral edema is considered a class effect, and should be proactively monitored. Exposure-response analyses of tepotinib indicate edema risk is associated with advanced age (31). Recommendations include measurement of weight and peripheral limbs at baseline to enable detection, and proactive management using support stockings, limb elevation, increased physical activity, and kinesiotherapy (15, 32). Diuretics can also be considered and may provide short-term relief; however, currently, treatment modifications are the most investigated mitigation strategies. Dose reductions, which can enable patients to continue benefiting from treatment, should be considered early to mitigate severity, and frequent short interruptions can also be considered. Data from a phase I study of tepotinib, and translational modelling approaches, indicate that ≥95% MET inhibition can be achieved at reduced doses of tepotinib (12, 33). ILD-like events were uncommon, although lung function monitoring remains important in patients being treated for NSCLC.
Overall, this updated analysis with detailed subgroup data from VISION provides a large dataset supporting the robust efficacy and tolerable safety profile of tepotinib in this patient population with METex14 skipping NSCLC, regardless of treatment setting.
Supplementary Material
Acknowledgments
The authors would like to thank patients and their families, investigators, co-investigators, and the study teams at all participating centers, as well as the healthcare business of Merck KGaA, Darmstadt, Germany. The trial was sponsored by the healthcare business of Merck KGaA, Darmstadt, Germany (CrossRef Funder ID: 10.13039/100009945).
Medical writing assistance (funded by the healthcare business of Merck KGaA, Darmstadt, Germany) was provided by Carys Davies, PhD, of Syneos Health, UK.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked advertisement in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
Note: Supplementary data for this article are available at Clinical Cancer Research Online (http://clincancerres.aacrjournals.org/).
Authors' Disclosures
X. Le reports grants and personal fees from EMD Serono and Eli Lilly and Company; personal fees from AstraZeneca, Hengrui Therapeutics, Daiichi Sanyko, Janssen, Novartis, AbbVie, and Spectrum Pharmaceutics; and grants and personal fees from Boehringer Ingelheim outside the submitted work. H. Sakai reports grants and personal fees from the healthcare business of Merck KGaA, Darmstadt, Germany, during the conduct of the study as well as personal fees from Ono Pharmaceutical Co., Bristol-Myers Squibb, AstraZeneca, Chugai Pharmaceutical Co., and Taiho Pharmaceutical Co. outside the submitted work. R. Veillon reports other support from the healthcare business of Merck KGaA, Darmstadt, Germany, during the conduct of the study as well as personal fees from Roche, AstraZeneca, BMS, Janssen, and Merck & Co. outside the submitted work. M.C. Garassino reports grants and personal fees from AstraZeneca and the healthcare business of Merck KGaA, Darmstadt, Germany, and personal fees from the healthcare business of Merck KGaA, Darmstadt, Germany, BMS, Roche, Daiichi Sankyo, Celgene, GSK, Eli Lilly and Company, Novartis, Regeneron, and Gilead during the conduct of the study as well as personal fees from AstraZeneca, Roche, Gilead, Merck & Co., BMS, Eli Lilly and Company, GSK, Regeneron, Novartis, and Celgene outside the submitted work. J. Raskin reports personal fees from Boehringer Ingelheim, Eli Lilly and Company, and Pfizer; non-financial support from Roche; and personal fees from BMS outside the submitted work. A.B. Cortot reports personal fees and non-financial support from AstraZeneca, Bristol-Myers Squibb, Merck & Co., and Pfizer; grants, personal fees, and non-financial support from Novartis and Roche; grants and personal fees from the healthcare business of Merck KGaA, Darmstadt, Germany; personal fees from Johnson & Johnson; and personal fees and non-financial support from Takeda outside the submitted work. S. Viteri reports personal fees from Takeda, the healthcare business of Merck KGaA, Darmstadt, Germany, BMS, Merck & Co., Janssen, Puma, AstraZeneca, and Roche outside the submitted work. J. Mazieres reports personal fees from the healthcare business of Merck KGaA, Darmstadt, Germany, during the conduct of the study as well as personal fees from Roche, AstraZeneca, BMS, Merck & Co., Takeda, and Novartis; grants from Pierre Fabre; and personal fees from Daiichi Sankyo outside the submitted work. E.F. Smit reports other support from the healthcare business of Merck KGaA, Darmstadt, Germany, during the conduct of the study. M. Thomas reports grants and personal fees from AstraZeneca, Bristol-Myers Squibb, Roche, and Takeda and personal fees from AbbVie, Amgen, Boehringer Ingelheim, Celgene, Chugai Pharma, Janssen Oncology, Eli Lilly and Company, Mirati Therapeutics, Bristol-Myers Squibb, BrightPath Biotherapeutics, Janssen Global Services, Nexus Health Systems, EMD Serono, Pneuma Respiratory, Kairos Venture Investments, Leads Biolabs, the healthcare business of Merck KGaA, Darmstadt, Germany, Merck & Co., Novartis, and Pfizer outside the submitted work. W.T. Iams reports personal fees from Genentech, Jazz Pharma, G1 Therapeutics, Mirati, OncLive, Clinical Care Options, Chardan, Outcomes Insights, Cello Health, and Curio Science outside the submitted work. B.C. Cho reports grants from Novartis, Bayer, AstraZeneca, MOGAM Institute, Dong-A ST, Champions Oncology, Janssen, Yuhan, Ono, Dizal Pharma, Merck & Co., AbbVie, Medpacto, GIInnovation, Eli Lilly and Company, Blueprint Medicines, and Interpark Bio Convergence Corp.; personal fees from Novartis, AstraZeneca, Boehringer Ingelheim, Roche, BMS, Ono Pharmaceutical, Yuhan, Pfizer, Eli Lilly and Company, Janssen, Takeda, Merck & Co., Medpacto, Blueprint Medicines, TheraCanVac Inc., Gencurix Inc., Bridgebio Therapeutics, KANAPH Therapeutic Inc., Cyrus Therapeutics, Interpark Bio Convergence Corp., Guardant Health, Joseah BIO, and Champions Oncology; and other support from DAAN Biotherapeutics outside the submitted work. J.C.-H. Yang reports other support from Amgen, Eli Lilly and Company, JNJ, and GSK; grants, personal fees, and other support from AstraZeneca; personal fees and other support from Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi Sankyo, the healthcare business of Merck KGaA, Darmstadt, Germany, Merck & Co., Novartis, Roche/Genentech, Takeda Oncology, and Yuhan Pharmaceutical; personal fees from Ono Pharmaceutical and Pfizer; and other support from Puma Technology outside the submitted work. J.D. Patel reports other support from AstraZeneca, Takeda, and Eli Lilly and Company outside the submitted work. C.M. Bestvina reports other support from EMD Serono during the conduct of the study as well as personal fees from AstraZeneca, Genentech, JNJ, Novartis, Pfizer, Seattle Genetics, and Takeda; grants and personal fees from BMS; and personal fees from Jazz Pharmaceuticals outside the submitted work. K. Park reports other support from the healthcare business of Merck KGaA, Darmstadt, Germany, outside the submitted work. F. Griesinger reports personal fees from the healthcare business of Merck KGaA, Darmstadt, Germany, during the conduct of the study as well as grants and personal fees from AstraZeneca, Boehringer Ingelheim, BMS, Merck & Co., Novartis, Pfizer, Roche, GSK, Siemens, Amgen, and Takeda; grants from Eli Lilly and Company; and personal fees from AbbVie outside the submitted work. M. Johnson reports grants and other support from the healthcare business of Merck KGaA, Darmstadt, Germany, during the conduct of the study as well as grants and other support from Mirati Therapeutics, AbbVie, Amgen, AstraZeneca, Atreca, Calithera Biosciences, Checkpoint Therapeutics, CytomX, Daiichi Sankyo, Eli Lilly and Company, EMD Serono, Genentech/Roche, Genmab, GlaxoSmithKline, Gritstone Oncology, Guardant Health, IDEAYA Biosciences, Incyte, Janssen, Loxo Oncology, Novartis, Pfizer, Regeneron Pharmaceuticals, Ribon Therapeutics, Sanofi, Turning Point Therapeutics, and WindMIL; grants from Acerta, Adaptimmune, Apexigen, Arcus Biosciences, Array BioPharma, Artios Pharma, BeiGene, BioAtla, Corvus Pharmaceuticals, Curis, Dracen Pharmaceuticals, Dynavax, Elicio Therapeutics, Erasca, Genocea Biosciences, Harpoon, Helsinn Healthcare SA, Hengrui Therapeutics, Hutchinson MediPharma, IGM Biosciences, Immunocore, Jounce Therapeutics, Kadmon Pharmaceuticals, Lycera, Memorial Sloan-Kettering, NeoImmune Tech, Neovia Oncology, Numab Therapeutics, OncoMed Pharmaceuticals, PMV Pharmaceuticals, RasCal Therapeutics, Relay Therapeutics, Revolution Medicines, Rubius Therapetics, Seven and Eight Biopharmaceuticals/Birdie Biopharmaceuticals, Shattuck Labs, Sillicon Therapeutics, Stem CentRx, Syndax Pharmaceuticals, Takeda Pharmaceuticals, Tarveda, TCR2 Therapeutics, Tempest Therapeutics, Tizona Therapeutics, TMUNITY Therapeutics, University of Michigan, Vyriad, and y-mAbs Therapeutics; and other support from Boehringer Ingelheim, Achilles Therapeutics, Axelia Oncology, Black Diamond, Bristol-Myers Squibb, EcoR1, Editas Medicine, Eisai, G1 Therapeutics, ITeos, and Oncorus outside the submitted work. C. Britschgi reports personal fees and non-financial support from AstraZeneca and Takeda and personal fees from Pfizer, Roche, Janssen-Cilag, and Boehringer Ingelheim outside the submitted work. J. Heymach reports grants and personal fees from AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, and Takeda Pharmaceuticals; personal fees from Genentech, Catalyst, Guardant Health, Foundation Medicine, Hengrui, Eli Lilly and Company, Novartis, Sanofi, Mirati Therapeutics, Bristol-Myers Squibb, BrightPath Biotherapeutics, Janssen Global Services, Nexus Health Systems, EMD Serono, Pneuma Respiratory, Kairos Venture Investments, and Leads Biolabs; grants, personal fees, and other support from Spectrum; and personal fees from RefleXion outside the submitted work. E. Sikoglu reports other support from the healthcare business of Merck KGaA, Darmstadt, Germany, during the conduct of the study. K. Berghoff and K.-M. Schumacher are employees of the healthcare business of Merck KGaA, Darmstadt, Germany. R. Bruns is an employee and stockholder of the healthcare business of Merck KGaA, Darmstadt, Germany. G. Otto is an employee of the healthcare business of Merck KGaA, Darmstadt, Germany, and holds stocks with Novartis. P.K. Paik reports personal fees and other support from EMD Serono during the conduct of the study; personal fees and other support from Calithera; personal fees from Takeda and Xencor; other support from Boehringer Ingelheim; and personal fees and other support from Bicara outside the submitted work. No disclosures were reported by the other authors.
Authors' Contributions
X. Le: Conceptualization, investigation, writing–review and editing. H. Sakai: Conceptualization, investigation, writing–review and editing. E. Felip: Conceptualization, investigation, writing–review and editing. R. Veillon: Conceptualization, investigation, writing–review and editing. M.C. Garassino: Conceptualization, investigation, writing–review and editing. J. Raskin: Conceptualization, investigation, writing–review and editing. A.B. Cortot: Conceptualization, investigation, writing–review and editing. S. Viteri: Conceptualization, investigation, writing–review and editing. J. Mazieres: Conceptualization, investigation, writing–review and editing. E.F. Smit: Conceptualization, investigation, writing–review and editing. M. Thomas: Conceptualization, investigation, writing–review and editing. W.T. Iams: Conceptualization, investigation, writing–review and editing. B.C. Cho: Conceptualization, investigation, writing–review and editing. H.R. Kim: Conceptualization, investigation, writing–review and editing. J.C.-H. Yang: Conceptualization, investigation, writing–review and editing. Y.-M. Chen: Conceptualization, investigation, writing–review and editing. J.D. Patel: Conceptualization, investigation, writing–review and editing. C.M. Bestvina: Conceptualization, investigation, writing–review and editing. K. Park: Conceptualization, investigation, writing–review and editing. F. Griesinger: Conceptualization, investigation, writing–review and editing. M. Johnson: Conceptualization, investigation, writing–review and editing. M. Gottfried: Conceptualization, investigation, writing–review and editing. C. Britschgi: Conceptualization, investigation, writing–review and editing. J. Heymach: Conceptualization, investigation, writing–review and editing. E. Sikoglu: Conceptualization, investigation, writing–review and editing. K. Berghoff: Conceptualization, methodology, writing–review and editing. K.-M. Schumacher: Conceptualization, methodology, writing–review and editing. R. Bruns: Conceptualization, data curation, formal analysis, methodology, writing–review and editing. G. Otto: Conceptualization, methodology, writing–review and editing. P.K. Paik: Conceptualization, investigation, writing–review and editing.
References
- 1. Awad MM, Oxnard GR, Jackman DM, Savukoski DO, Hall D, Shivdasani P, et al. MET exon 14 mutations in non-small-cell lung cancer are associated with advanced age and stage-dependent MET genomic amplification and c-Met overexpression. J Clin Oncol 2016;34:721–30. [DOI] [PubMed] [Google Scholar]
- 2. Frampton GM, Ali SM, Rosenzweig M, Chmielecki J, Lu X, Bauer TM, et al. Activation of MET via diverse exon 14 splicing alterations occurs in multiple tumor types and confers clinical sensitivity to MET inhibitors. Cancer Discov 2015;5:850–9. [DOI] [PubMed] [Google Scholar]
- 3. Paik P, Drilon A, Fan P-DD, Yu H, Rekhtman N, Ginsberg MS, et al. Response to MET inhibitors in patients with stage IV lung adenocarcinomas harboring met mutations causing exon 14 skipping. Cancer Discov 2015;5:842–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tong JH, Yeung SF, Chan AWH, Chung LY, Chau SL, Lung RWM, et al. MET amplification and exon 14 splice site mutation define unique molecular subgroups of non-small cell lung carcinoma with poor prognosis. Clin Cancer Res 2016;22:3048–56. [DOI] [PubMed] [Google Scholar]
- 5. Sabari JK, Leonardi GC, Shu CA, Umeton R, Montecalvo J, Ni A, et al. PD-L1 expression, tumor mutational burden, and response to immunotherapy in patients with MET exon 14 altered lung cancers. Ann Oncol 2018;29:2085–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Digumarthy SR, Mendoza DP, Zhang EW, Lennerz JK, Heist RS. Clinicopathologic and imaging features of non-small-cell lung cancer with MET exon 14 skipping mutations. Cancers 2019;11:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ali A, Goffin JR, Arnold A, Ellis PM. Survival of patients with non-small-cell lung cancer after a diagnosis of brain metastases. Curr Oncol 2013;20:e300–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Awad MM, Leonardi GC, Kravets S, Dahlberg SE, Drilon A, Noonan SA, et al. Impact of MET inhibitors on survival among patients with non-small cell lung cancer harboring MET exon 14 mutations: a retrospective analysis. Lung Cancer 2019;133:96–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Offin M, Luo J, Guo R, Lyo JK, Falcon C, Dienstag J, et al. CNS metastases in patients with MET exon 14–altered lung cancers and outcomes with crizotinib. JCO Precis Oncol 2020;4:871–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bittoni M, Yang JCH, Shih JY, Peled N, Smit E, Camidge DR, et al. Real-world insights into patients (pts) with advanced NSCLC and MET alterations. Ann Oncol 2020;31:S1386–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cai B, Zhou Z-Y, Xue W, Hazra NC, Singh M, Mishra D, et al. Budget impact of capmatinib for adults with metastatic non-small cell lung cancer harboring a MET exon 14 skipping mutation in the United States. J Med Econ 2021;24:131–9. [DOI] [PubMed] [Google Scholar]
- 12. Falchook GS, Kurzrock R, Amin HM, Xiong W, Fu S, Piha-Paul SA, et al. First-in-man Phase I trial of the selective MET inhibitor tepotinib in patients with advanced solid tumors. Clin Cancer Res 2020;26:1237–46. [DOI] [PubMed] [Google Scholar]
- 13. Markham A. Tepotinib: first approval. Drugs 2020;80:829–33. [DOI] [PubMed] [Google Scholar]
- 14. Viteri S, Mazieres J, Veillon R, Felip E, Le X, Garassino MC, et al. Activity of tepotinib in brain metastases (BM): Preclinical models and clinical data from patients (pts) with MET exon 14 (METex14) skipping NSCLC. Ann Oncol 2020;31:S754–840. [Google Scholar]
- 15. Paik P, Felip E, Veillon R, Sakai H, Cortot AB, Garassino MC, et al. Tepotinib in non-small-cell lung cancer with MET exon 14 skipping mutations. N Engl J Med 2020;383:931–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hanna NH, Schneider BJ, Temin S, Baker S, Brahmer J, Ellis PM, et al. Therapy for stage IV non–small-cell lung cancer without driver alterations: ASCO and OH (CCO) joint guideline update. J Clin Oncol 2020;38:1608–32. [DOI] [PubMed] [Google Scholar]
- 17. National Comprehensive Cancer Network. Non-small cell lung cancer version 4.2021. 2021[cited 2021 Mar 18]. Available from: https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf.
- 18. Japanese Lung Cancer Society. Lung cancer practice guidelines. 2020. [cited 2021 May 13]. Available from: https://www.haigan.gr.jp.
- 19. Le X, Heymach JV. New verse for a familiar song: small molecule inhibitors for MET exon 14 skipping non-small cell lung cancer. Oncologist 2020;25:822–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. FDA. TEPMETKO Prescribing Information. 2021.
- 21. FDA. Center for drug evaluation and research (application number: 214096Orig1s000): multi-discipline review. 2020[cited 2021 Mar 17]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2021/214096Orig1s000MultidisciplineR.pdf.
- 22. Wolf J, Seto T, Han J-Y, Reguart N, Garon EB, Groen HJM, et al. Capmatinib in MET exon 14–mutated or MET -amplified non–small-cell lung cancer. N Engl J Med 2020;383:944–57. [DOI] [PubMed] [Google Scholar]
- 23. Drilon A, Clark JW, Weiss J, Ou SHI, Camidge DR, Solomon BJ, et al. Antitumor activity of crizotinib in lung cancers harboring a MET exon 14 alteration. Nat Med 2020;26:47–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Vansteenkiste JF, Smit EF, Groen HJM, Garon EB, Heist RS, Hida T, et al. Capmatinib in patients with METex14-mutated advanced non-small cell lung cancer who received prior immunotherapy: The phase II GEOMETRY mono-1 study. Ann Oncol 2020;31:S830. [Google Scholar]
- 25. Dagogo-Jack I, Moonsamy P, Gainor JF, Lennerz JK, Piotrowska Z, Lin JJ, et al. A phase 2 study of capmatinib in patients with MET-altered lung cancer previously treated with a MET inhibitor. J Thorac Oncol 2021;16:850–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Takamori S, Matsubara T, Fujishita T, Ito K, Toyozawa R, Seto T, et al. Dramatic intracranial response to tepotinib in a patient with lung adenocarcinoma harboring MET exon 14 skipping mutation. Thorac Cancer 2021;12:978–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Roth KG, Mambetsariev I, Salgia R. Prolonged survival and response to tepotinib in a non-small-cell lung cancer patient with brain metastases harboring MET exon 14 mutation: a research report. Cold Spring Harb Mol Case Stud 2020;6:a005785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tanaka H, Taima K, Makiguchi T, Nakagawa J, Niioka T, Tasaka S. Activity and bioavailability of tepotinib for leptomeningeal metastasis of NSCLC with MET exon 14 skipping mutation. Cancer Commun 2021;41:83–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lu S, Fang J, Cao L, Li X, Guo Q, Zhou J, et al. Preliminary efficacy and safety results of savolitinib treating patients with pulmonary sarcomatoid carcinoma (PSC) and other types of non-small cell lung cancer (NSCLC) harboring MET exon 14 skipping mutations [abstract]. In: Proceedings of the AACR Annual Meeting; 2019 Mar 29–Apr 3; Atlanta, GA. Philadelphia (PA): AACR; 2019. Abstract nr CT031.
- 30. Le Rhun E, Guckenberger M, Smits M, Dummer R, Bachelot T, Sahm F, et al. EANO-ESMO clinical practice guidelines for diagnosis, treatment and follow-up of patients with brain metastasis from solid tumours. Ann Oncol 2021;32:1332–47. [DOI] [PubMed] [Google Scholar]
- 31. Paik P, Xiong W, Hietala SF, Nyberg J, Papasouliotis O, Anziano R, et al. 584P Tepotinib exposure-response analyses of safety and efficacy in patients with solid tumours. Ann Oncol 2020;31:S494–5. [Google Scholar]
- 32. Goodwin K, Ledezma B, Heist R, Garon E. Management of selected adverse events with capmatinib: Institutional experiences from the GEOMETRY Mono-1 trial [abstract]. IASLC2021Jan 1; 16(1 Suppl S16–S17). Abstract nr MO01.04.
- 33. Xiong W, Friese-Hamim M, Johne A, Stroh C, Klevesath M, Falchook GS, et al. Translational pharmacokinetic-pharmacodynamic modeling of preclinical and clinical data of the oral MET inhibitor tepotinib to determine the recommended phase II dose. CPT Pharmacometrics Syst Pharmacol 2021;10:428–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Any requests for data by qualified scientific and medical researchers for legitimate research purposes will be subject to the healthcare business of Merck KGaA, Darmstadt, Germany, Data Sharing Policy. All requests should be submitted in writing to the healthcare business of Merck KGaA, Darmstadt, Germany, data sharing portal (https://www.emdgroup.com/en/research/our-approach-to-research-and-development/healthcare/clinical-trials.html).