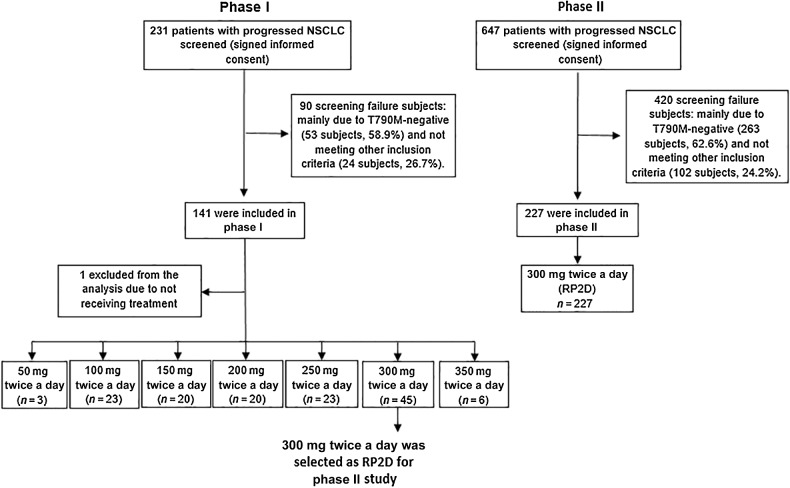
Figure 1.
Study profile. The phase I population comprised all patients who received at least one dose of abivertinib and had a baseline RECIST assessment in 7 doses: 3 patients treated with 50 mg twice a day, 23 patients treated with 100 mg twice a day, 20 patients treated with 150 mg twice a day, 20 patients treated with 200 mg twice a day, 23 patients treated with 250 mg twice a day, 45 patients treated with 300 mg twice a day, 6 patients treated with 350 mg twice a day, and 1 patient without receiving Abivertinib treatment was excluded from the study. The phase II was enrolled 227 patients treated with RP2D. Note: Not meeting other inclusion criteria included laboratory examination requirements, status of brain metastasis and prior treatment, etc.