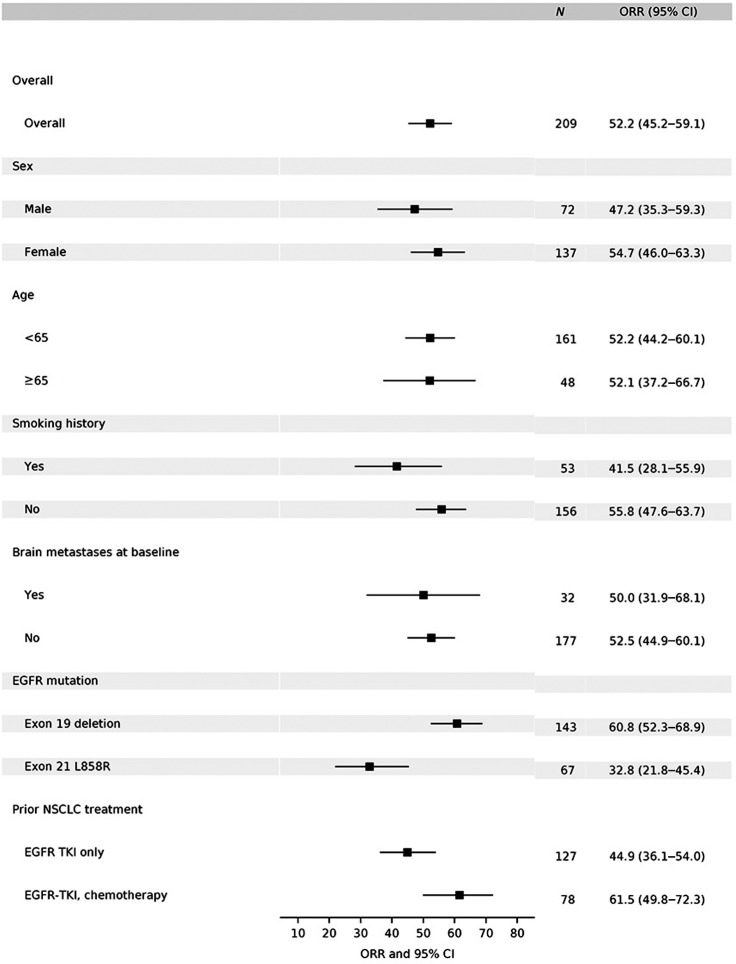
Figure 3.
Subgroup analyses per investigator assessment in the response–evaluable set. Subgroups analysis of overall objective response in evaluable patients in phase II by investigator assessment. EGFR mutation status was determined by central test. Brain metastases at screening was determined by ≥1 brain lesion. Note: For EGFR mutation subgroup analysis, 3 subjects who had both Exon 19 deletion and L858R mutations included in both subgroups; 2 subjects who had neither Exon 19 deletion nor L858R mutations not included in both subgroups; for prior NSCLC treatment subgroup analysis, 2 subjects who had chemotherapy only and 2 subjects who have neither EGFR-TKI nor chemotherapy not included in both subgroups.