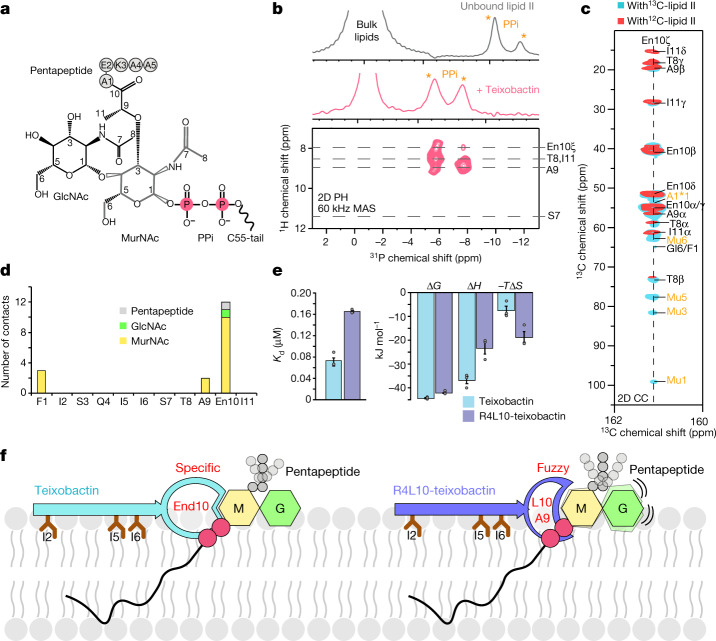
Fig. 3. Enduracididine governs the interface.
a, Chemical structure of lipid II. b, 1D 31P ssNMR data in liposomes show strong shifts of the lipid II PPi signals upon addition of teixobactin. 2D 1H31P (HP) ssNMR shows a direct interaction between the PPi group and the backbone amino-protons of the depsi-cycle and the sidechain of End10. MAS, magic angle spinning. c, Superposition of 2D 13C13C (CC) spectra of the complex with NMR-invisible 12C,14N-labelled (red) and NMR-active 13C,15N-lipid II (cyan) show a dominant presence of enduracididine at the interface. The cyan spectrum was acquired with a magnetization transfer time of 300 ms. Interfacial contacts with MurNAc and A1 of the pentapeptide are shown in orange. d, Sum of interfacial ssNMR contacts with the headgroup of lipid II. e, Binding energetics of the interface obtained by isothermal titration calorimetry. Data show the averages of three experiments for each drug. Data represented as mean ± s.e.m. Source data are provided as a Source Data file. f, Illustration of the differential binding modes of natural teixobactin (left) and the synthetic analogue R4L10-teixobactin18. Enduracididine in teixobactin specifically binds to MurNAc, which stabilizes the entire interface. The substitution of End10 by a leucine residue leads to a fuzzy interface with the headgroup of lipid II and reduces the binding affinity.