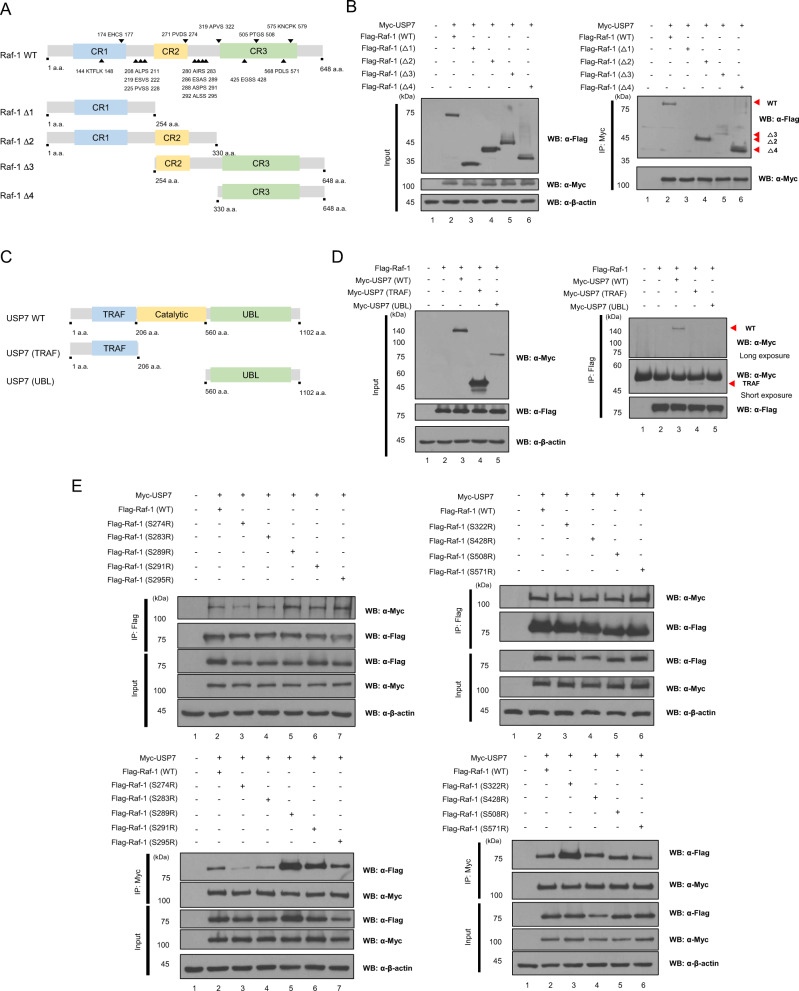
Fig. 2. The PVSS motif belonging to the catalytic domain of Raf-1 binds to proteins containing the ubiquitin-like (Ubl) domain of USP7.
A The deletion mutants and two binding motif sites ([P/A/E]-X-X-S and K-X-X-X-K) of Raf-1 are indicated by the schematic drawing. Raf-1 ∆1 contains only the CR1 domain and Raf-1 ∆2 contains the CR1 and CR2 domains. Raf-1 ∆3 contains the CR2 and CR3 domains, and Raf-1 ∆4 contains only the CR3 domain (Raf-1 ∆1:1 a.a.-254 a.a., Raf-1 ∆2:1 a.a.-330 a.a., Raf-1 ∆3: 254 a.a.-648 a.a., and Raf-1 ∆4: 330 a.a.-648 a.a.). B HEK293T cells were both transfected with Myc-USP7 and four different Flag-Raf-1 deletion mutants. And then immunoprecipitation assay using deletion mutants of Raf-1 was performed with an anti-Myc antibody. C The deletion mutants of USP7 are indicated by the schematic drawing. USP7 (TRAF) contains only the TRAF domain of USP7 and USP7 (UBL) contains only the UBL domain (USP7 (TRAF): 1 a.a.-104 a.a. and USP7 (UBL): 562 a.a.-1103 a.a.). D Flag-Raf-1 and two different Myc-USP7 deletion mutants were transfected into HEK293T cells and then immunoprecipitation assay was performed with an anti-Flag antibody. E The binding motif mutants of Raf-1 (S274R, S283R, S289R, S291R, S295R, S322R, S428R, S508R, and S571R) are generated by site-directed mutagenesis and Myc-USP7 were co-transfected into HEK293T cells. And then immunoprecipitations were performed with an anti-Myc antibody.