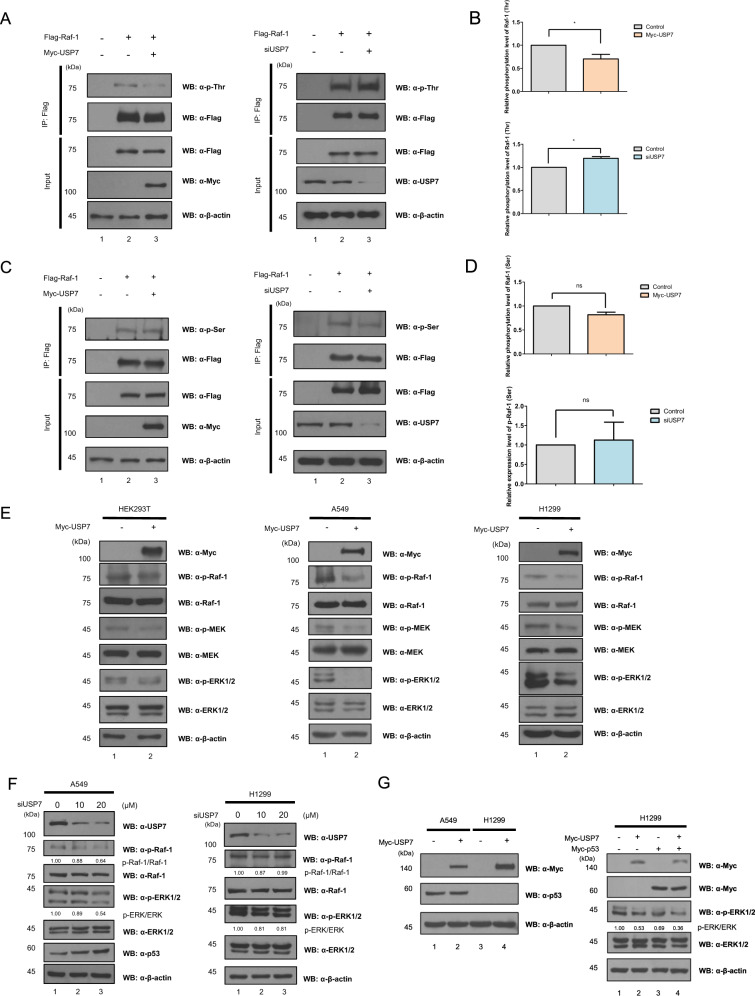
Fig. 4. USP7 regulates the ERK1/2 signaling pathway by reducing the phosphorylation level of Raf-1.
A Flag-Raf-1 and Myc-USP7 or siUSP7 were transfected into HEK293T cells and Flag-Raf-1 was precipitated by an anti-Flag antibody. Threonine phosphorylation of Raf-1 was detected by Western blotting using an anti-p-Thr antibody. B Threonine phosphorylation level of Flag-Raf-1 in at least three independent experiments was calculated by a two-tailed Student’s t-test, *p < 0.05, n = 5 (left), n = 3 (right). C Flag-Raf-1 and Myc-USP7 or siUSP7 were transfected into HEK293T cells and Flag-Raf-1 was precipitated by an anti-Flag antibody. Threonine phosphorylation of Raf-1 was detected by Western blotting using an anti-p-Ser antibody. D Serine phosphorylation level of Flag-Raf-1 in at least three independent experiments was calculated by a two-tailed Student’s t-test, ns = not significant, n = 3 (left), n = 3 (right). E The expression of the ERK1/2 signaling factors (Raf-1, MEK1/2, and ERK1/2) and their active forms (p-Raf-1, p-MEK1/2, and p-ERK1/2) were detected by Western blotting using indicated antibodies in HEK293T, A549, and H1299 cells. The experiment was performed at least three times and representative data were shown. F siUSP7 was transfected into A549 and H1299 cells in a dose-dependent manner. The expression of USP7, p53, Raf-1, p-Raf-1, ERK1/2, and p-ERK1/2 was detected by Western blotting. G Myc-USP7 was transfected into A549 and H1299 cells. The expression of Myc-USP7, and p53 was detected by Western blotting, and a partial deletion form of p53 protein in H1299 cells was also confirmed by Western blotting using an anti-p53 antibody. Myc-USP7 was transfected with or without Myc-p53 in H1299 cells. The expression of ERK1/2 and p-ERK1/2 was detected by Western blotting. The experiment was performed at least three times and representative data were shown.