Abstract
Obesity is one of the major pandemics of the 21st century. Due to its multifactorial etiology, its treatment requires several actions, including dietary intervention and physical exercise. Excessive fat accumulation leads to several health problems involving alteration in the gut-microbiota-brain axis. This axis is characterized by multiple biological systems generating a network that allows bidirectional communication between intestinal bacteria and brain. This mutual communication maintains the homeostasis of the gastrointestinal, central nervous and microbial systems of animals. Moreover, this axis involves inflammatory, neural, and endocrine mechanisms, contributes to obesity pathogenesis. The axis also acts in appetite and satiety control and synthesizing hormones that participate in gastrointestinal functions. Exercise is a nonpharmacologic agent commonly used to prevent and treat obesity and other chronic degenerative diseases. Besides increasing energy expenditure, exercise induces the synthesis and liberation of several muscle-derived myokines and neuroendocrine peptides such as neuropeptide Y, peptide YY, ghrelin, and leptin, which act directly on the gut-microbiota-brain axis. Thus, exercise may serve as a rebalancing agent of the gut-microbiota-brain axis under the stimulus of chronic low-grade inflammation induced by obesity. So far, there is little evidence of modification of the gut-brain axis as a whole, and this narrative review aims to address the molecular pathways through which exercise may act in the context of disorders of the gut-brain axis due to obesity.
Keywords: microbiota-gut-brain axis, exercise, obesity, gut-derived peptides, dysbiosis
Introduction
The obesity epidemic has reached over 2 billion people worldwide, with 39% of the world population being overweight. This number is expected to increase to 50% by 2030 (1). Obesity has multifactorial pathogenesis and is associated with pathologies characterized by metabolic disorders, such as type II diabetes (2, 3). In addition, obesity is associated with increased risk of stress, depression, anxiety, decreased satiety, and reduction of life expectancy (1). On the other hand, dietary control and increased energy expenditure through physical activity have been used as the main weight-reduction strategies (4, 5).
Obesity has been commonly associated with dysregulation of intestinal function, altered gut microbiota, and appetite dysregulation (6, 7). These physiologic responses are closely related, involving the gut microbiota, the gastrointestinal tract, and the brain, which compose the microbiota-gut-brain axis (MGB axis) (8). For example, a report on lean animals that for two weeks received a transplant of the fecal microbiota from obese animals led to a significant increase in body weight (9). More recently, studies have indicated that physical activity could attenuate the physiologic outcomes of obesity, which may be associated with a modulation of the MGB-axis (10–12). According to the literature, sedentary hypertensive animals (SHR) that received a transplant of fecal microbiota from SHR animals that performed physical exercise had attenuated systolic blood pressure and a change in the gut-brain axis through the modulation of the gut microbiota (13). It is believed that different exercise training variables (e.g., intensity, volume, type of exercise) may influence neurotransmitter signaling involved in appetite control, intestinal integrity, permeability, and alteration of the gut microbiota (14–16).
Although these responses have never been investigated collectively in a single study, it is believed that the modulation of the MGB-axis by physical activity can result in antagonistic reactions compared to changes due to obesity (7, 10, 17–19). Moderate exercise has been associated with improved gut health, intestinal permeability control, increased microbial variation, and appetite regulation (17, 20, 21). On the other hand, obesity is often associated with antagonistic characteristics such as increased intestinal permeability (leaky gut), dysbiosis, and appetite dysregulation (7, 22, 23). In this context, the present review will address the molecular mechanisms involved in modulating the MGB-axis by physical exercise and obesity and their contrasting points.
Microbiota-gut-brain axis
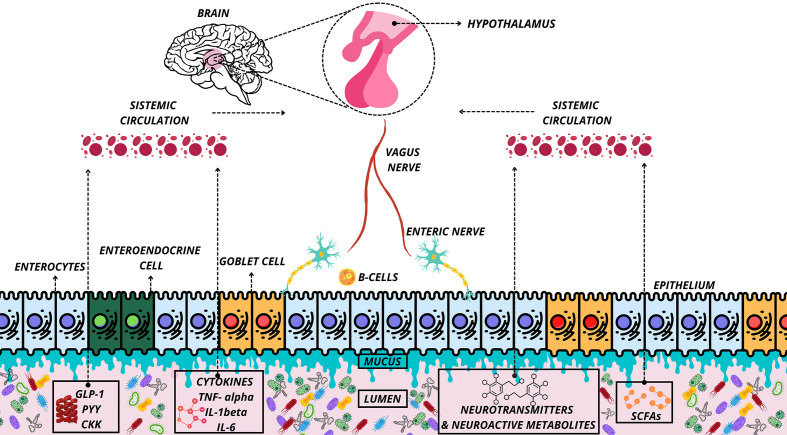
The foremost communicators between the brain and the gut (MGB-axis) are the central nervous system (CNS), the enteric nervous system (ENS), the autonomic nervous system (ANS), and the hypothalamic-pituitary-adrenal (HPA) axis (24), see Figure 1 . The common feature of the MGB axis is the inclusion of gut microbes, metabolites, and gut peptides in gut-brain bidirectional communication (24, 25). The vagus nerve (NV) mediates communication between the gut and the brain (24). However, this communication can also occur indirectly, though microbial-derived intermediaries such as short-chain fatty acids (SCFAs), secondary bile acids (2BAs), tryptophan metabolites, and cytokines (interleukin-6, IL-6) (24).
Figure 1.
Organization of the microbiota-gut-brain axis. Representation of communication of the microbiota-gut-brain axis under normal conditions. Release of neurotransmitters and neuroactive metabolites, cytokines, peptides and SCFAs in the systemic circulation and delivery of these substances to the interacting tissues and hypothalamus. SCFAs, Short-Chain Fatty Acid; PYY, Peptide YY; GLP-1, Glucagon Like Peptide-1; CKK, Cholecystokinin; IL, interleukin.
Each component of the MGB axis communicates bi-directionally within the ANS, antagonistically and synergistically (24). Excess adipose tissue is associated with changes in both sympathetic and parasympathetic activities (26). However, the decrease in body weight can reverse the changes in ANS caused by obesity (27). Thus, the ANS seems crucial for a better understanding of the pathophysiology of obesity (28).
Another critical factor related to the MGB-axis is the immune system (29). Low-grade chronic inflammation is a common feature of metabolic diseases such as obesity and an increased factor in developing neurological conditions (30). In addition, low-grade chronic systemic inflammation is associated with dysbiotic microbiota and malfunctioning immune responses (31, 32). In this regard, it has been shown that microbial-derived SCFAs seem to impair the proper functioning of microglia, brain macrophages responsible for antigen presentation, phagocytosis, and modulating inflammation throughout life (33–35).
Since the MGB-axis includes components directly involved with the nervous system, such as the metabolism and hormonal and immune systems, dysfunctions between its features may result in negative impacts on the host’s health (36). Not all the mechanisms by which training acts on the MGB axis are explicit. Here, the role of the MGB axis in the pathogenesis of obesity will be discussed, and whether physical activity (or physical training) could benefit the axis and treat obesity from a chronic perspective.
The impact of obesity on the microbiota-gut-brain axis
Deregulation of the MGB axis is associated with several metabolic and neurologic pathologies, such as Alzheimer’s, Parkinson’s, and obesity (37–39). After food consumption, sensory information crosses the NV and is sent to the nucleus tractus solitarius (NTS). NTS neurons integrate the incoming vagal information with another neuroendocrine signal into the hypothalamus (40). Energy balance signaling in the hypothalamus (via NTS neurons) can recognize changes in dietary pattern (41). For example, increased chronic intake of hypercaloric diets can modulate the communication of the NS pathway, which can cause a hormonal imbalance related to appetite control, leading the individual to obesity (41, 42).
The hypothalamus is considered the “command center” of satiety and energy expenditure (42). Changes in the hypothalamus signaling will reflect on the received stimulus (43). In this regard, obesity can dysregulate several peptides or their receptors that are known to decrease food intake, such as nesfatin-1, oxyntomodulin (OXM), CCK, glucagon-like peptide 1 (GLP-1), pancreatic polypeptide (PP), and PYY (44), as shown in Table 1 . By changing these molecules, obesity leads to deficient signaling to the hypothalamus, causing hypothalamic dysfunction and energy imbalance (62, 63).
Table 1.
Functions of hormones/peptides and possible changes due to obesity.
| Hormone / peptide | Secreting body | Function | Contributing factor | Influence of obesity | Author |
|---|---|---|---|---|---|
| Ghrelin | Stomach | Meal starter; long-term regulation of body weight; energy fuel division. | Hypercaloric / hyperlipidic diet | ↑ Levels and acceleration of gastric emptying | (45) |
| Peptide YY (PYY) | Intestine | Meal inhibitor; ↑ satiety; ↑ intestinal motility | Snack hypercaloric 2000 kcal | ↓ Plasma PYY after meal and fasting | (46) |
| Glucagon like peptide-1 (GLP-1) | Large intestine | ↑ In the release of insulin; inhibition of gastric emptying and secretion of gastric acid in the stomach; ↑ satiety in the brain; | Liraglutide | Suppression in the concentrations of GLP-1 | (47) |
| Cholecystokinin (CCK) | Small intestine | Stimulates the contraction of the gallbladder; ↑ satiety; ↑ the secretion of pancreatic enzymes for digestion of carbohydrates, proteins and fats; | – | ↓ CCK release, stimulating ghrelin secretion. | (48) |
| Pancreatic polypeptide (PP) | Pancreas | ↑ Energy expenditure; ↑ satiety; suppression of pancreatic secretion; stimulation of gastric secretion; | Hypercaloric / hyperlipidic diet | ↓PP | (49) |
| Oxytomodulin (OXM) | Small intestine | ↑ Energy expenditure; ↑ satiety; suppression of pancreatic secretion; stimulation of gastric secretion; | Infusion of PYY and OXM | ↓ OXM. Infusions result in ↓ energy intake. | (50) |
| Gastic inhibitor polypeptide or glucose-dependent insulinotropic (gip) | Large intestine | Inhibits water absorption; ↑ stimulating lipase. | High-fat diet | ↑ GIP concentration: ↑ visceral and hepatic fat, ↑ blood flow in adipose tissue; | (51) |
| Gastrin | Small intestine | ↑ Intestinal motility; stimulates the growth of the intestinal mucosa; | High-fat diet | ↓ Gastrin, weight gain. | ( (52) |
| Leptin | Stomach | Control of energy intake; ↑ satiety; | High-fat diet | ↑ Circulating levels, resistance to its capture. | (53) |
| Adiponectin | Blood flow | Glycemia regulation; fatty acid catabolism; ↑ insulin sensitization; | Thiazolidinediones or CB1 antagonists (rimonabant) increase a plastic adiponectin | ↓ Adiponectinemia, contributing to the pathogenesis of insulin resistance, type 2 diabetes, cardiovascular disease in obese or overweight people | (54) |
| Insulina | Adipose tissue/pancreas | ↓ Blood glucose control; lipid storage | High-fat diet | Insulin resistance, ↓ the body's glucose uptake | (55) |
| Neuro peptide Y (NPY) | Adipose tissue | ↑ In energy storage; ↑ in food intake; | Hypercaloric / hyperlipidic diet | ↓ Levels, triggering weight gain | (56) |
| Melanocortin | Adipose tissue | Energy balance regulation | – | ↑ Melanocortin and the MC4R gene | (57) |
| Islet amyloid polypeptide (IAPP) or amylin | Stomach | Gastric acid secretion; inhibition of gastric emptying; release of glucagon; ↓ of food intake; ↓ weight gain and adiposity | – | Plasma levels are ↑ in obese individuals | (58) |
| Orexin or hypocretin | Stomach/ intestine | Regulation of intestinal motility; regulation in pancreatic secretion; regulation of food intake; | Hyperlipidemic diet | ↓ In plasma levels, which can ↓ energy expenditure. | (59) |
| Visfatin (VF) | Adipose tissue visceral | Glucose regulation; insulin-like action; | Hyperlipidemic diet | ↓ Plasma concentrations, triggering ↓ glucose sensitivity | (60) |
| Nesfatin-1 | Hypothalamus | Appetite regulator; energy homeostasis regulator; | Hyperlipidemic diet | In obese people the concentration is ↑, ↑ food intake ↓ satiety; | (61) |
↑ - increase and greater; ↓ - decrease and decline;
(↑) Increase Secretion and Greater; (↓) Decrease Secretion and Decline.
The high caloric consumption in the Western diet can cause an inflammatory environment in the digestive tract associated with microbiome disturbances (64). In this sense, saturated long-chain fats can activate toll-like receptors 4 (TRL4) and initiate an inflammatory process in astrocytes, microglia, and neurons (65). Inflammation of the hypothalamus is characterized by exacerbated proliferation of glial cells, infiltration of microglia, and proliferation of astrocytes (65, 66). Hypothalamic inflammation caused by obesity generates mitochondrial dysfunction (62). The melanocortin system consists of several critical neuronal populations that participate in hypothalamic mitochondrial regulation (67) and are located in the agouti-related protein (AgRP)/neuropeptide Y (NPY) and proopiomelanocortin (POMC)/cocaine- and amphetamine-regulated transcript (CART) neurons ( Figure 2 ). In response to food consumption, the α-melanocyte-stimulating hormone (α-MSH) is released from POMC/CART-expressing neurons. It binds to melanocortin receptors 3 and 4 (MC3/4R), reducing appetite and increasing energy expenditure (68). The opposite occurs with AgRP/NPY-expressing neurons, which release AgRP neuropeptides that bind to MC4R and inhibit POMC neurons, stimulating hunger and decreasing energy expenditure (68). Thus, several studies have sought to understand MC4R signaling pathways due to their importance in regulating appetite and obesity (69–71).
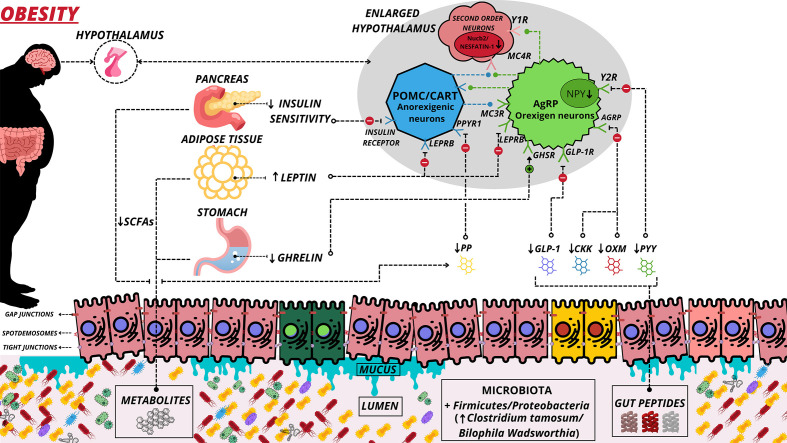
Figure 2.
Alteration of the microbiota-gut-brain axis in obesity. Main hormonal changes derived from obesity. Obesity leads to damage to epithelial cells and damage to gap junctions of these cells, which allows greater permeability of undesirable substances to the systemic circulation. A decrease in mucus and decline in the interactions of some peptides/hormones with their respective receptors also occurs. Red cells illustrate inflamed cells. (↑) Increase Secretion and Greater; (↓) Decrease Secretion and Decline; (⊕) Positive interaction; (⊖) Negative Interaction; (⊘) Non Interaction; SCFAs, Short-Chain Fatty Acid; PYY, Peptide YY; OXM, Oxytomodulin; PPYR1, Pancreatic Polypeptide Receptor 1; PP, Pancreatic Polypeptide; GLP-1, Glucagon Like Peptide-1; GLP-1R, Glucagon Like Peptide-1 Receptor; LEPRB, Leptin Receptor Long Isoform; Y1R, Neuropeptide Y Receptor type 1; Y2R, - Neuropeptide Y Receptor type 2; GHSR, - Growth Hormone Secretagogue receptor; CKK, Cholecystokinin; MC3R, Melanocortin 3 Receptor; MC4R, Melanocortin 4 Receptor; AgRP, Agouti-Related Protein.
Nesfatin-1 is an anorectic neuropeptide associated with appetite regulation, malnutrition, and weight reduction (see Figure 2 ). The reduction of nesfatin-1 has been identified in overweight and obese children, adolescents, and adults (72, 73). Nesfatin-1 is derived from nucleobindin-2 (Nucb2) mRNA. Nucb2 reduction is also identified in obese people; interestingly, this reduction can lead to insulin resistance (74). Recently, it was identified that Nucb2/Nesfatin-1 is reduced in the hypothalamus of obese individuals (75). Also, an increase in nesfatin-1 in the brain leads to activation of the insulin receptor (InsR)/insulin receptor substrate-1 (IRS-1), increasing insulin sensitivity (76). Thus, this peptide appears to be a target for regulating appetite and glycemic control (77, 78).
Several peptides can be altered due to obesity (79). Enteroendocrine cells (EEC) release the hormone GLP-1, which acts on gastric reduction, satiety control, and decreased apoptosis of pancreatic beta cells (80). GLP-1 is reduced in obese people (81). It was recently identified that applying subcutaneous injections of GLP-1 receptor agonist exenatide 2 mg (ExQW) once a week and over 36 weeks leads to a reduction in the total adipose tissue waist circumference of obese individuals (82). In this context, the pharmacological manipulation of GLP-1 receptor agonists as a target in taste perception and weight loss has recently emerged (47, 83). PYY and cholecystokinin (CCK) peptides are also related to appetite control and decreased gastric secretion (84, 85). In obese individuals, PYY and CCK are usually reduced (45, 46). Animals with the inhibited CCK receptor (knockout model) tend to acquire obesity and develop non-insulin-dependent diabetes mellitus (86). Interestingly, these animals also contain an elevation of neuropeptide Y (NPY) mRNA expression in the dorsomedial hypothalamic (DMH) area (86). This peptide increases appetite and is commonly overexpressed in obese people (87). PYY and NPY are similar peptides sharing the same receptors (Y1-Y3 and Y5 receptors) (88), as shown in Figure 2 . Obesity increases peripheral NPY in adipose tissue macrophages with autocrine and paracrine signals (89). Besides, adipose Y5R mRNA is higher in obese than non-obese individuals (90). Thus, a drug induction strategy with antagonistic effects of neuropeptide receptors has emerged as an anti-obesity treatment (91, 92).
Ghrelin and leptin are other peptides that significantly impact satiety control ( Table 1 ). These two hormones are related to food intake and body weight (93). Ghrelin is an orexigenic hormone that acts on the hypothalamus’s arcuate nucleus (Arc) in response to fasting. Ghrelin stimulates the GH secretion of growth hormone (GH) by the GH secretagogue-receptor (GHS-R). Obese people have low ghrelin levels and leptin resistance (lower leptin receptor expression, Lep-R) (94, 95). A higher circulating leptin level is considered a marker of uncontrolled eating in these individuals.
Furthermore, as a result of ghrelin reduction, obese people also have a GH deficiency (96). Recently, it has been identified that the synthetic GHSR agonist (hexarelin) reduces fat accumulation and improves insulin sensitivity in obese mice (97). Although drug treatments for obesity have shown promise, they are not yet effective in slowing the disease progression and require multiple health domains extending beyond weight reduction (98). Fat accumulation leads to intestinal, hypothalamic, and systemic inflammation (99, 100). Excessive triglyceride in fat cells increases the release of tumor necrosis factor-alpha (TNF-α) and pro-inflammatory interleukins and decreases the expression of anti-inflammatory molecules such as adiponectin (101). These pro-inflammatory adipokines participate in the increase of systemic and intestinal inflammation (102, 103).
Furthermore, gut-derived peptide disturbances are also related to increased intestinal inflammation caused by obesity (104). The derived inflammatory signaling from obesity is associated with anatomic and physiologic changes in the intestine. The mucosa layer is composed of epithelial cells (enterocytes) connected by specialized proteins knowns as tight junctions (TJ) (105). These proteins are responsible for “filtering” the components that are absorbed by the intestinal enterocytes (105). An increase in TNF-α and IL-13 decreases TJ expression, increasing the chances of intestinal inflammation. Also, an increase in TJ in blood circulation is associated with the deleterious effects of obesity on intestinal integrity (106, 107). Treatments with peptides such as CCK can preserve the intestinal mucosa’s integrity and decrease TJ dysfunction (104). Furthermore, the gut microbiota is an essential component of TJ control, intestinal mucosa, and satiety regulation (108).
Several studies have shown that obese phenotypes are associated with the altered composition and low abundance of the gut microbiota (109–111). Gut microbiota can ferment indigestible fibers and produce SCFAs (109). In this sense, animals that ate a high-fat diet containing 10% fermentable flaxseed fiber, which increased total SCFA levels, gained less weight than those that ate without the fiber (112). These results agree with the SFCA’s being able to mediate the energy balance of obesity by increasing energy expenditure and fat oxidation (113). SCFAs can also protect adipocytes from leukocyte infiltration by attenuating interleukin-1β (IL-1β) and TNF-α expression, in addition to restoring the adiponectin production in high-fat-fed mice (114). Furthermore, SCFAs appear to be the “bridge” of communication between the gut microbiota and the brain (115). Due to this communication, the gut microbiota can regulate inflammation in the hypothalamus and is believed to be one of the avenues of appetite control and obesity treatment (116).
More recently, high BMI was associated with lower alpha diversity; however, the gut microbiota from obesogenic phenotypes may vary according to race/ethnicity, dietary components, or socioeconomic status (117). Moreover, some bacteria such as F. prausnitzii, R. faecis, A. muciniphila, Prevotelaceae, and Ruminococcus have been associated with weight reduction (118, 119). More recently, Akkermansia muciniphila was shown to reduce gut barrier disruption and insulin resistance (120), where individuals with diabetes and obesity present a reduced abundance of this species, leading to some prospects in treating obesity (121). Moreover, obese mice supplemented with SCO-792, an available enteropeptidase inhibitor reported to have therapeutic effects on obesity and diabetes, increased the abundance of A. muciniphila (122). Besides, an increase in Prevotella in overweight adults has been related to significant weight reduction (123). Thus, the gut microbiota seems to participate in the brain-intestine axis due to the functions in the host’s metabolism and may play a role in treating obesity by regulating appetite (124, 125).
Obesity is also associated with immunological changes throughout the MGB axis (126, 127). Adipose tissue is considered an endocrine organ and secretes some proinflammatory proteins (adipokines), such as leptin, resistin, and angiopoietin-like protein 2 (ANGPTL2) (128). Leptin and ANGPTL2 stimulate the activation and proliferation of monocytes and macrophages (129, 130). Resistin drives inflammation by elevating TNF-α and IL-6, activating the Toll-like receptor (TLR) 4-affiliated pro-inflammatory pathway and developing insulin resistance (131). Excess adipose tissue can lead to these immune and metabolic changes (132, 133).
During obesity, the protective interleukins (IL-17-producing Th17 cells, IL-10-secreting regulatory T (Treg) cells, and IL-22) are reduced (127, 134), while there is a more significant release of pro-inflammatory cytokines such as tumor necrosis factor (TNF-α) and interferon (IFNγ). This results in damage to the gut barrier expressed by reduced expression of epithelial tight junction proteins and antimicrobial proteins such as regenerating islet-derived protein 3 gamma (RegIIIγ) (135). This excessive permeability in the intestine is termed “leaky gut” and allows for translocation of bacteria products, triggering “metabolic endotoxemia” and systemic inflammation (136). Furthermore, some bacterial species of the microbiota, such as A. muciniphila, Bifidobacterium pseudocatenulatum CECT 7765, and B. uniformis CECT 7771, can act to elevate Treg cells, prevent B cell infiltration in fat, and reduce B cells and the M1/M2 macrophage ratio (137–139). The ingestion of these species alleviates obesity (139–141).
The studies presented here indicate the MGB axis as a complementary target for treating obesity due to its direct participation in controlling food satiety, macronutrient absorption, and inflammatory processes (39, 142). Despite preliminary evidence, further studies are needed, especially to highlight the impact of each element of the axis on the pathogenesis of obesity and the effect of this multifactorial disease on these target organs. Moreover, it is still necessary to investigate how different interventions can influence the MGB axis, such as dietary interventions, sleep, life stages, and physical activity.
The impact of physical activity on the microbiota-gut-brain axis
Muscle contraction in response to physical exercise promotes a series of acute and chronic physiological changes in the organism, many of which are associated with disease prevention and health improvement (143). Muscle contraction through exercise increases energy demand on muscle fibers, and the supply to vital organs is altered (144). Blood suppression in the gastrointestinal system depends on the intensity of the exercise. While mild-to-moderate exercise can preserve mucosal and improve intestinal motility, high-intensity exercise is associated with epithelial injury, enhanced permeability, reduced gastric motility, and other imbalances (144). These physiological changes in the intestine also generate several molecular changes in the MGB axis ( Figure 3 ). Thus, it has been hypothesized that controlled physical training can improve intestine health, increase microbial diversity and abundance, and alter neurotransmitters that regulate appetite (17).
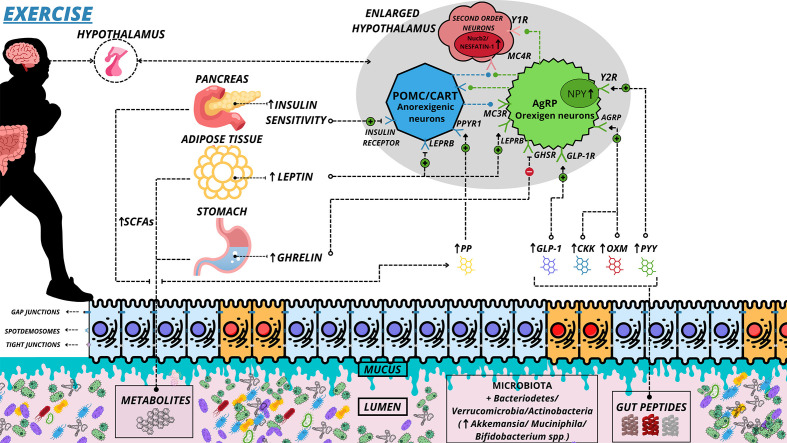
Figure 3.
Alteration of the microbiota-gut-brain axis in exercise. Main hormonal changes in response to physical exercise. Exercise can maintain the health of epithelial cells, and cell communications remain healthy, without permeability of substances to systemic circulation. Mucus preservation and improved interaction of peptides/hormones with their receptors also occur, creating optimal conditions. Blue cells represent healthy. (↑) Increase Secretion and Greater; (↓) Decrease Secretion and Decline; (⊕) Positive interaction; (⊖) Negative Interaction; (⊘) Non Interaction; SCFAs, Short-Chain Fatty Acid; PYY, Peptide YY; OXM, Oxytomodulin; PPYR1, Pancreatic Polypeptide Receptor 1; PP, Pancreatic Polypeptide; GLP-1, Glucagon Like Peptide-1; GLP-1R, Glucagon Like Peptide-1 Receptor; LEPRB, Leptin Receptor Long Isoform; Y1R, Neuropeptide Y Receptor type 1; Y2R, - Neuropeptide Y Receptor type 2; GHSR, - Growth Hormone Secretagogue receptor; CKK, Cholecystokinin; MC3R, Melanocortin 3 Receptor; MC4R, Melanocortin 4 Receptor; AgRP, Agouti-Related Protein.
The role of exercise in appetite regulation related to obesity may be approached by investigating the acute effect of exercise or its chronic responses (14, 145). Acute exercise suppresses acylated ghrelin and increases GLP-1 and PYY, which could be associated with satiety control (145). The temporary suppression of appetite occurs around 60% of the VO2 peak (146–150) and has been shown in different types of exercise, such as running (146, 147, 149), cycling (148, 151, 152), swimming (153), high-intensity interval exercise (154, 155) and resistance training (156) (see Table 2 ). However, peptide signaling may vary according to the exercise intensity and volume, diet, temperature, trainability, and the period of the day the exercise is performed (18, 154, 174–177).
Table 2.
Possible changes by acute and chronic exercise in hormones/peptides that participate in MGB axis.
| Hormone / peptide | Subjects | Exercise Type | Exercise Intensity | Exercise Volume | Contributing Factor | Changes by exercise | Author |
|---|---|---|---|---|---|---|---|
| Ghrelin | Healthy - 7 W and 6 M (n = 13) | Acute cycling | 70% VO2 peak | 60 min | Ketone monoester drink or dextrose control isocaloric drink | ↓ Ghrelin levels after exercise | (157) |
| Peptide YY (PYY) | Healthy - 7 W and 6 M (n = 13) | Acute cycling | 70% VO2 peak | 60 min | Ketone monoester drink or dextrose control isocaloric drink | There was no significant difference in total PPY. ↑ PYY3-36 in high-intensity exercise | (157, 158) |
| Glucagon like peptide-1 (GLP-1) | Healthy – M (n=10) | Acute cycling | high intensity session: 75% VO2 max, moderate intensity session: 50% VO2 max | 30 min, 3x week | instant noodles and a piece of cheese: 532 kcal, 13.9% protein, 26.6% fat, and 59.5% carbohydrate | ↑ GLP-1 after exercise 30 min exercise | (157) |
| Cholecystokinin (CCK) | Sedentary obese M (n= | Chronic Aerobic | 75% FCmax | 5x wk/ 12 wks | 500-kcal energy deficit per session | There was no significant change after chronic exercise intervention | (159) |
| Pancreatic Polypeptide (PP) | Sedentary obese - M and W (n=13) | Aerobic | 75% VO2 peak (2weeks) | 60 min | 1.500 kcal intake for 12 h (6 meals every 2 h) | ↑ Fasting PP after 15 days of exercise | (160) |
| Oxytomodulin (OXM) | Healthy W and M (n=15) | Aerobic | (HIE) 70% VO2 max (MIE) 50%VO2 max | HIE = 20min MIE = 30min | – | ↑ Oxyntomodulin after chronic aerobic exercise only in the HIE group | (161) |
| Gastric Inhibitor Polypeptide OR glucose-dependent insulinotropic (GIP) | pre-diabetic and obese W and M (n=22) | Chronic aerobic | 85% FCmax | 60 min 5x wk/ 12 wks |
High glycemic index diet / low glycemic index diet. | The group with a low glycemic index diet showed ↓ GIP compared to the group with a high glycemic index. | (162) |
| Gastrin | Wistar rats M (n=24) | Swimming | – | 30 min | 50% food restriction | ↑ Gastrin and improvement of intestinal hormonal dysfunction | (163) |
| Leptin | Adolescent obese W and M (n=72) | Combined training; Aerobic training and physical leisure | – | 60 min | 6 months | ↓ Leptin levels and reduced resistance | (164) |
| Adiponectin | Healthy W and M (n=29) | Combined training | 60-70% cardiac reserve and 80% 1RM | 20 min | – | Adiponectin ↑ 55% after exercise and there was a ↑ in post-exercise compared to the control group. | (165) |
| Insulina | Healthy W and M (n=32) | Cycling | 60-80% FCmax/ 60-80 RPM | – | Isocaloric diet | ↑ Sensitivity; ↓ insulin secretion; | (166) |
| Neuro peptide Y (NPY) | Athletes (n=12) | Paddle ergometer and Resistance training | 40-50% RM | 15h/20h for week | High carbohydrate diet | The NPY values in the exercise were significantly ↑ immediately after and after 30 minutes. | (167) |
| Melanocortin | Overweight to obese and postmenopausal W (n=23) | Resistance training | 8 RM, and resistance until muscle failure | – | "Normal" diet throughout the intervention period and do not consume alcohol in the days before any blood collection. | Resistance training can modulate the expression of the melanocortin 3 receptor | (168) |
| Islet amyloid polypeptide (IAPP) or Amylin | Healthy M (n=7) | Incremental test on the treadmill | 60, 75, 90, 100% VO2 max | 10, 10, 5, 2 min | Without alcohol 24h before the test | ↑ Amylin levels in well-trained individuals | (169) |
| Orexin or Hypocretin | Healthy M (n=10) | Cycling ergometric | 75w and 60 RPM | 15 min | Without strenuous physical activity 7 days and without medication, alcohol or coffee | Thermoregulator during exercise; appetite control; | (170) |
| Visfatin (VF) | Sedentary W (n=48) | Combined Training | 40% increased 60-75% FC máx | 45 min + 20 min | – | physical training and weight loss can ↓ visfatin levels | (171) |
| Visfatin (VF) | Healthy M (n=6) | Aerobic | 7 sets of 6 × 35 m every 10 s, with 1 min rest between sets) | 45 min | – | ↑ visfatin levels plasma | (172) |
| Nesfatin-1 | Overweight W with metabolic syndrome (n=60) | (EA) aerobic exercises; (ER) resistance exercises; (EC) combined exercises | (EA): 60-75% FCmax; (ER): 60 Increased 75 - 80% 1RM; (EC): EA and ER simultaneous | 30 and 60 min | No changes in habits | Nesfatin-1 ↑ significantly after physical training in the three intervention groups. | (173) |
↑ - increase or gain; ↓ - reduction or loss; FCmax, Maximum Heart Rate; W, Woman; M, Male; min, minutes; n =, sample; wk/wks, week/week; HIE, High Intensity Exercise; MIE, Moderate Intensity Exercise.
(↑) Increase Secretion and Greater; (↓) Decrease Secretion and Decline; (wks) Weeks; (min) Minute.
An experiment with an animal model showed that ghrelin levels increase after an acute bout of exercise, where this response was dependent on running distance or time (174). In addition, animals with low ghrelin receptors (GHSR-nulls) decreased endurance performance and food intake following high-intensity interval exercise (174). It was also shown that the CCK increases after acute exercise, which optimizes the satiety state (178). Moreover, healthy women submitted to sensitive high-intensity training presented increased levels of GLP-1 and a reduction in hunger compared to moderate exercise (155). On the other hand, the effects of activity on the MGB axis appear to be even more consistent (14). Physical training plays an anorectic role that seems to be enhanced with training, increasing leptin levels, glucose insulinotropic peptide (GIP), nestin-1, adiponectin, GLP-1, PP, OXM, and PYY ( Figure 3 and Table 2 ). To date, no research has analyzed the changes of all these peptides simultaneously in the context of physical exercise.
Despite the replication in several modalities on appetite control, aerobic training seems more effective than resistance in increasing the satiety of overweight and obese adults (179). However, in overweight and sedentary individuals, it has recently been observed that 12 weeks of resistance training decreased ghrelin and PYY concentrations more than the proposed aerobic protocol (180). These data demonstrate no consensus concerning the training modality to reduce overweight people’s appetite. Exercise is also able to change the functional anatomy characteristics of the intestine. Physical activity alone increased the thickness, height of villi, and the rats’ crypts’ depth submitted to a hypothalamic obesity condition (181). Exercise is also able to alter intestinal integrity through TJ (182). Some evidence shows that physical training increases the expression of zonulin, claudin, and occluding proteins (TJs), in addition to decreasing the concentration of circulating lipopolysaccharides (LPS), thus having a protective effect on the intestinal barrier (183), see Figure 3 . However, intensity and volume determine the beneficial effect of exercise on intestinal permeability (144). More than 60 min of vigorous endurance training at 70% of the maximum work capacity led to increased intestinal permeability (144). Thus, depending on the applied dose of exercise, exercise can generate an antagonistic effect of obesity on the brain-intestine axis (11).
It has been known for a few years that exercise can also alter gut microbiota composition (15, 184). Some of these alterations include increased bacterial richness (α-diversity), butyrate-producing bacteria, and the abundance of A. muciniphila and Faecalibacterium prausnitzii (15, 185, 186). In obese children, the combination of 12 weeks of strength and endurance training was shown to neutralize changes in the microbiota caused by obesity, reducing the Proteobacteria phylum and Gammaproteobacteria class (187). This training protocol also increased the Blautia, Dialister, and Roseburia genera and the abundance of SCFA, leading to a similar status observed in healthy children (187). A recent study in overweight and obese adults showed that long-term training (6 months) demonstrated subtle microbiota changes and no relationship between alpha diversity and cardiorespiratory fitness or fat mass (19). In overweight older adults, regular exercise reshaped microbial composition and function alterations induced by aging (16). It is worth mentioning that the positive action of exercise on the microbiota and immune system depends on the intensity and volume of training and the individual’s trainability (188).
Physical exercise may also influence the MGB axis in pathophysiological contexts through bidirectional communication between the muscle, the intestine, and the brain (muscle-gut-brain axis) (188, 189). Skeletal muscle can act as an endocrine organ and release into the bloodstream molecules (PYY, irisin, myonectin, and others) called myokines (190, 191). There is some evidence that these myokines may act on appetite and changes in the gut microbiota (190, 192, 193). The skeletal muscle proteomic profile identified more than 300 myokines and these molecules perform various functions in the body, such as lipid and glucose metabolism, browning of white fat, bone formation, endothelial cell function, etc (191). The myokines IL-6, IL-7, IL-15 and leukemia inhibitory factor (LIF) also exert immune functions (194). In this sense, resistance training plus aerobic can increase the obese animals’ IL-7 expression (195). IL-7 is a vital myokine responsible for lymphocyte homeostasis and body fat reduction (196). Furthermore, since the IL-15/sIL-15Rα gene transfer induced weight loss in obese animals (197), IL-15 is estimated to be a potential regulator of fat mass (198). Interestingly, obese mice trained for 12 weeks on a treadmill increased IL-15 mRNA expression and IL-15 immunoreactivity in muscle (199). Thus, further clinical studies are expected to better explain how muscle communicates with the immune system, gut, brain and gut microbiota in the context of obesity.
Conclusion and prospects
The current scientific literature presents a body of evidence indicating that obesity contributes to increased inflammatory signaling in the hypothalamus and increased appetite and gastric motility, in addition to being associated with enterocyte lesions and contributing to dysbiosis development ( Figure 2 and Table 1 ). However, regular physical activity has an anti-inflammatory effect on the hypothalamus and regulates appetite by increasing anorexigenic peptides (leptin, GIP, nesfatin-1, adiponectin, GLP-1, PP, OXM, and PYY). Moreover, the thickness, height of villi, and depth of crypts improve intestinal integrity through tight junctions and reduce the impact of obesity on the gut microbiota ( Figure 3 and Table 2 ).
Current evidence initially points to an antagonistic response promoted by exercise and obesity in the MGB-axis (157, 181, 187). However, despite initially presenting antagonistic effects, physical exercise can adversely affect the gastrointestinal system and its associated microbiota, mainly when performed in larger training volumes and hot environments with little hydration, as previously reviewed (144). Nevertheless, the above conclusions have been drawn from different clinical studies and, in several cases using animal models, as there is still no study aiming to combine all the MGB axis elements.
In this context, further studies are needed to identify the antagonistic elements and mechanisms promoted by physical exercise and obesity in the MGB axis. Although some “anti-obese” drugs have emerged, these drugs are ineffective in treating obesity (200). Thus, future studies that analyze these drugs added to a physical training program are interesting. Furthermore, the exercise dose-response must also be further investigated, considering its different modalities and variations in intensity and volume in healthy and obese individuals. Perhaps, more important than identifying the opposite signals promoted by both stimuli is to understand how exercise can mitigate and reverse the adverse effects of obesity through the modulation of the MGB axis.
Author contributions
FR: Participated in the writing of the article and produced the figures and tables. MS: Prepared the tables. VL: Prepared the tables. HL: Participated in the report of the paper. GM: Prepared the figures. OF: Participated in the writing and review of the paper. BP; Participated in the writing and study of the article. All authors contributed to the article and approved the submitted version.
Funding
This research was supported by CNPq (437308/2018-9), CAPES, FUNDECT: 134789-2014, and FAPDF: 357489-2020.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1. Andolfi C, Fisichella PM. Epidemiology of obesity and associated comorbidities. J Laparoendosc Adv Surg Tech A (2018) 28:919–24. doi: 10.1089/lap.2018.0380 [DOI] [PubMed] [Google Scholar]
- 2. Wilding JPH, Mooney V, Pile R. Should obesity be recognised as a disease? BMJ (2019) 366:l4258. doi: 10.1136/bmj.l4258 [DOI] [PubMed] [Google Scholar]
- 3. Al-Goblan AS, Al-Alfi MA, Khan MZ. Mechanism linking diabetes mellitus and obesity. Diabetes Metab Syndr Obes (2014) 7:587–91. doi: 10.2147/DMSO.S67400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gorostegi-Anduaga I, Corres P, MartinezAguirre-Betolaza A, Perez-Asenjo J, Aispuru GR, Fryer SM, et al. Effects of different aerobic exercise programmes with nutritional intervention in sedentary adults with overweight/obesity and hypertension: EXERDIET-HTA study. Eur J Prev Cardiol (2018) 25:343–53. doi: 10.1177/2047487317749956 [DOI] [PubMed] [Google Scholar]
- 5. Fock KM, Khoo J. Diet and exercise in management of obesity and overweight. J Gastroenterol Hepatol (2013) 28 Suppl 4:59–63. doi: 10.1111/jgh.12407 [DOI] [PubMed] [Google Scholar]
- 6. Kim KN, Yao Y, Ju SY. Short chain fatty acids and fecal microbiota abundance in humans with obesity: A systematic review and meta-analysis. Nutrients (2019) 11(10):251. doi: 10.3390/nu11102512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bliss ES, Whiteside E. The gut-brain axis, the human gut microbiota and their integration in the development of obesity. Front Physiol (2018) 9:900. doi: 10.3389/fphys.2018.00900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Frank J, Gupta A, Osadchiy V, Mayer EA. Brain-Gut-Microbiome interactions and intermittent fasting in obesity. Nutrients (2021) 13(2):584. doi: 10.3390/nu13020584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature (2006) 444:1027–31. doi: 10.1038/nature05414 [DOI] [PubMed] [Google Scholar]
- 10. Dalton A, Mermier C, Zuhl M. Exercise influence on the microbiome-gut-brain axis. Gut Microbes (2019) 10:555–68. doi: 10.1080/19490976.2018.1562268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Royes LFF. Cross-talk between gut and brain elicited by physical exercise. Biochim Biophys Acta Mol Basis Dis (2020) 1866:165877. doi: 10.1016/j.bbadis.2020.165877 [DOI] [PubMed] [Google Scholar]
- 12. Oppert JM, Bellicha A, van Baak MA, Battista F, Beaulieu K, Blundell JE, et al. Exercise training in the management of overweight and obesity in adults: Synthesis of the evidence and recommendations from the European association for the study of obesity physical activity working group. Obes Rev (2021) 22 Suppl 4:e13273. doi: 10.1111/obr.13273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Xia WJ, Xu ML, Yu XJ, Du MM, Li XH, Yang T, et al. Antihypertensive effects of exercise involve reshaping of gut microbiota and improvement of gut-brain axis in spontaneously hypertensive rat. Gut Microbes (2021) 13:1–24. doi: 10.1080/19490976.2020.1854642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dorling J, Broom DR, Burns SF, Clayton DJ, Deighton K, James LJ, et al. Acute and chronic effects of exercise on appetite, energy intake, and appetite-related hormones: The modulating effect of adiposity, sex, and habitual physical activity. Nutrients (2018) 10(9):1140. doi: 10.3390/nu10091140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mitchell CM, Davy BM, Hulver MW, Neilson AP, Bennett BJ, Davy KP. Does exercise alter gut microbial composition? A Systematic Review Med Sci Sports Exerc (2019) 51:160–7. doi: 10.1249/MSS.0000000000001760 [DOI] [PubMed] [Google Scholar]
- 16. Zhu Q, Jiang S, Du G. Effects of exercise frequency on the gut microbiota in elderly individuals. Microbiologyopen (2020) 9:e1053. doi: 10.1002/mbo3.1053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Martins C, Morgan L, Truby H. A review of the effects of exercise on appetite regulation: an obesity perspective. Int J Obes (Lond) (2008) 32:1337–47. doi: 10.1038/ijo.2008.98 [DOI] [PubMed] [Google Scholar]
- 18. Crabtree DR, Blannin AK. Effects of exercise in the cold on ghrelin, PYY, and food intake in overweight adults. Med Sci Sports Exerc (2015) 47:49–57. doi: 10.1249/MSS.0000000000000391 [DOI] [PubMed] [Google Scholar]
- 19. Kern T, Blond MB, Hansen TH, Rosenkilde M, Quist JS, Gram AS, et al. Structured exercise alters the gut microbiota in humans with overweight and obesity-a randomized controlled trial. Int J Obes (Lond) (2020) 44:125–35. doi: 10.1038/s41366-019-0440-y [DOI] [PubMed] [Google Scholar]
- 20. Aragon-Vela J, Solis-Urra P, Ruiz-Ojeda FJ, Alvarez-Mercado AI, Olivares-Arancibia J, Plaza-Diaz J. Impact of exercise on gut microbiota in obesity. Nutrients (2021) 13(11):3999. doi: 10.3390/nu13113999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Keirns BH, Koemel NA, Sciarrillo CM, Anderson KL, Emerson SR. Exercise and intestinal permeability: another form of exercise-induced hormesis? Am J Physiol Gastrointest Liver Physiol (2020) 319:G512–8. doi: 10.1152/ajpgi.00232.2020 [DOI] [PubMed] [Google Scholar]
- 22. Person H, Keefer L. Psychological comorbidity in gastrointestinal diseases: Update on the brain-gut-microbiome axis. Prog Neuropsychopharmacol Biol Psychiatry (2021) 107:110209. doi: 10.1016/j.pnpbp.2020.110209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ley RE, Backhed F, Turnbaugh P, Lozupone CA, Knight RD, Gordon JI. Obesity alters gut microbial ecology. Proc Natl Acad Sci U S A (2005) 102:11070–5. doi: 10.1073/pnas.0504978102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cryan JF, O'Riordan KJ, Cowan CSM, Sandhu KV, Bastiaanssen TFS, Boehme M, et al. The microbiota-Gut-Brain axis. Physiol Rev (2019) 99:1877–2013. doi: 10.1152/physrev.00018.2018 [DOI] [PubMed] [Google Scholar]
- 25. Wikoff WR, Anfora AT, Liu J, Schultz PG, Lesley SA, Peters EC, et al. Metabolomics analysis reveals large effects of gut microflora on mammalian blood metabolites. Proc Natl Acad Sci U S A (2009) 106:3698–703. doi: 10.1073/pnas.0812874106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Valensi P. Autonomic nervous system activity changes in patients with hypertension and overweight: role and therapeutic implications. Cardiovasc Diabetol (2021) 20:170. doi: 10.1186/s12933-021-01356-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Costa J, Moreira A, Moreira P, Delgado L, Silva D. Effects of weight changes in the autonomic nervous system: A systematic review and meta-analysis. Clin Nutr (2019) 38:110–26. doi: 10.1016/j.clnu.2018.01.006 [DOI] [PubMed] [Google Scholar]
- 28. Guarino D, Nannipieri M, Iervasi G, Taddei S, Bruno RM. The role of the autonomic nervous system in the pathophysiology of obesity. Front Physiol (2017) 8:665. doi: 10.3389/fphys.2017.00665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fung TC. The microbiota-immune axis as a central mediator of gut-brain communication. Neurobiol Dis (2020) 136:104714. doi: 10.1016/j.nbd.2019.104714 [DOI] [PubMed] [Google Scholar]
- 30. Kim J, Yoon S, Lee S, Hong H, Ha E, Joo Y, et al. A double-hit of stress and low-grade inflammation on functional brain network mediates posttraumatic stress symptoms. Nat Commun (2020) 11:1898. doi: 10.1038/s41467-020-15655-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Larsen JM. The immune response to prevotella bacteria in chronic inflammatory disease. Immunology (2017) 151:363–74. doi: 10.1111/imm.12760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zheng D, Liwinski T, Elinav E. Interaction between microbiota and immunity in health and disease. Cell Res (2020) 30:492–506. doi: 10.1038/s41422-020-0332-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nayak D, Roth TL, McGavern DB. Microglia development and function. Annu Rev Immunol (2014) 32:367–402. doi: 10.1146/annurev-immunol-032713-120240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Nohr MK, Egerod KL, Christiansen SH, Gille A, Offermanns S, Schwartz TW, et al. Expression of the short chain fatty acid receptor GPR41/FFAR3 in autonomic and somatic sensory ganglia. Neuroscience (2015) 290:126–37. doi: 10.1016/j.neuroscience.2015.01.040 [DOI] [PubMed] [Google Scholar]
- 35. Nastasi C, Candela M, Bonefeld CM, Geisler C, Hansen M, Krejsgaard T, et al. The effect of short-chain fatty acids on human monocyte-derived dendritic cells. Sci Rep (2015) 5:16148. doi: 10.1038/srep16148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Schachtle MA, Rosshart SP. The microbiota-Gut-Brain axis in health and disease and its implications for translational research. Front Cell Neurosci (2021) 15:698172. doi: 10.3389/fncel.2021.698172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Megur A, Baltriukiene D, Bukelskiene V, Burokas A. The microbiota-Gut-Brain axis and alzheimer's disease: Neuroinflammation is to blame? Nutrients (2020) 13(1):37. doi: 10.3390/nu13010037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bullich C, Keshavarzian A, Garssen J, Kraneveld A, Perez-Pardo P. Gut vibes in parkinson's disease: The microbiota-Gut-Brain axis. Mov Disord Clin Pract (2019) 6:639–51. doi: 10.1002/mdc3.12840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Torres-Fuentes C, Schellekens H, Dinan TG, Cryan JF. The microbiota-gut-brain axis in obesity. Lancet Gastroenterol Hepatol (2017) 2:747–56. doi: 10.1016/S2468-1253(17)30147-4 [DOI] [PubMed] [Google Scholar]
- 40. Martin CR, Osadchiy V, Kalani A, Mayer EA. The brain-Gut-Microbiome axis. Cell Mol Gastroenterol Hepatol (2018) 6:133–48. doi: 10.1016/j.jcmgh.2018.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mohammadi M, Khodarahmi M, Kahroba H, Farhangi MA. Dietary patterns interact with the variations of 18q21.23 rs17782313 locus on regulation of hypothalamic-pituitary axis hormones and cardio-metabolic risk factors in obesity. Eat Weight Disord (2020) 25:1447–59. doi: 10.1007/s40519-020-00855-1 [DOI] [PubMed] [Google Scholar]
- 42. Ahima RS, Antwi DA. Brain regulation of appetite and satiety. Endocrinol Metab Clin North Am (2008) 37:811–23. doi: 10.1016/j.ecl.2008.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Mullins CA, Gannaban RB, Khan MS, Shah H, Siddik MAB, Hegde VK, et al. Neural underpinnings of obesity: The role of oxidative stress and inflammation in the brain. Antioxidants (Basel) (2020) 9(10):1018. doi: 10.3390/antiox9101018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ghanemi A, Yoshioka M, St-Amand J. Obesity as a neuroendocrine reprogramming. Medicina (Kaunas) (2021) 57(1):66. doi: 10.3390/medicina57010066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Steinert RE, Feinle-Bisset C, Asarian L, Horowitz M, Beglinger C, Geary N, et al. GLP-1, and PYY(3-36): Secretory controls and physiological roles in eating and glycemia in health, obesity, and after RYGB. Physiol Rev (2017) 97:411–63. doi: 10.1152/physrev.00031.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. le Roux CW, Batterham RL, Aylwin SJ, Patterson M, Borg CM, Wynne KJ, et al. Attenuated peptide YY release in obese subjects is associated with reduced satiety. Endocrinology (2006) 147:3–8. doi: 10.1210/en.2005-0972 [DOI] [PubMed] [Google Scholar]
- 47. Kim SH, Abbasi F, Nachmanoff C, Stefanakis K, Kumar A, Kalra B, et al. Effect of the glucagon-like peptide-1 analogue liraglutide versus placebo treatment on circulating proglucagon-derived peptides that mediate improvements in body weight, insulin secretion and action: A randomized controlled trial. Diabetes Obes Metab (2021) 23:489–98. doi: 10.1111/dom.14242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Mesgari-Abbasi M, Abbasalizad Farhangi M. Serum concentrations of cholecystokinin, peptide YY, ghrelin and high sensitive c-reactive protein in association with metabolic syndrome ingredients in obese individuals. Acta Endocrinol (Buchar) (2020) 16:37–42. doi: 10.4183/aeb.2020.37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Khandekar N, Berning BA, Sainsbury A, Lin S. The role of pancreatic polypeptide in the regulation of energy homeostasis. Mol Cell Endocrinol (2015) 418 Pt 1:33–41. doi: 10.1016/j.mce.2015.06.028 [DOI] [PubMed] [Google Scholar]
- 50. Field BC, Wren AM, Peters V, Baynes KC, Martin NM, Patterson M, et al. PYY3-36 and oxyntomodulin can be additive in their effect on food intake in overweight and obese humans. Diabetes (2010) 59:1635–9. doi: 10.2337/db09-1859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Thondam SK, Cuthbertson DJ, Wilding JPH. The influence of glucose-dependent insulinotropic polypeptide (GIP) on human adipose tissue and fat metabolism: Implications for obesity, type 2 diabetes and non-alcoholic fatty liver disease (NAFLD). Peptides (2020) 125:170208. doi: 10.1016/j.peptides.2019.170208 [DOI] [PubMed] [Google Scholar]
- 52. Mhalhal TR, Washington MC, Newman KD, Heath JC, Sayegh AI. Combined gastrin releasing peptide-29 and glucagon like peptide-1 reduce body weight more than each individual peptide in diet-induced obese male rats. Neuropeptides (2018) 67:71–8. doi: 10.1016/j.npep.2017.11.009 [DOI] [PubMed] [Google Scholar]
- 53. Myers MG, Jr., Leibel RL, Seeley RJ, Schwartz MW. Obesity and leptin resistance: distinguishing cause from effect. Trends Endocrinol Metab (2010) 21:643–51. doi: 10.1016/j.tem.2010.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Guerre-Millo M. Adiponectin: an update. Diabetes Metab (2008) 34:12–8. doi: 10.1016/j.diabet.2007.08.002 [DOI] [PubMed] [Google Scholar]
- 55. Shen J, Obin MS, Zhao L. The gut microbiota, obesity and insulin resistance. Mol Aspects Med (2013) 34:39–58. doi: 10.1016/j.mam.2012.11.001 [DOI] [PubMed] [Google Scholar]
- 56. Wu Y, He H, Cheng Z, Bai Y, Ma X. The role of neuropeptide y and peptide YY in the development of obesity via gut-brain axis. Curr Protein Pept Sci (2019) 20:750–8. doi: 10.2174/1389203720666190125105401 [DOI] [PubMed] [Google Scholar]
- 57. Girardet C, Butler AA. Neural melanocortin receptors in obesity and related metabolic disorders. Biochim Biophys Acta (2014) 1842:482–94. doi: 10.1016/j.bbadis.2013.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Lutz TA. Effects of amylin on eating and adiposity. Handb Exp Pharmacol (2012) 209:231–50. doi: 10.1007/978-3-642-24716-3_10 [DOI] [PubMed] [Google Scholar]
- 59. Mishra S, Gupta V, Mishra S, Sachan R, Asthana A. Serum level of orexin-a, leptin, adiponectin and insulin in north Indian obese women. Diabetes Metab Syndr (2017) 11 Suppl 2:S1041–3. doi: 10.1016/j.dsx.2017.07.037 [DOI] [PubMed] [Google Scholar]
- 60. Stastny J, Bienertova-Vasku J, Vasku A. Visfatin and its role in obesity development. Diabetes Metab Syndr (2012) 6:120–4. doi: 10.1016/j.dsx.2012.08.011 [DOI] [PubMed] [Google Scholar]
- 61. Huang X, Yang Z. Resistin's, obesity and insulin resistance: the continuing disconnect between rodents and humans. J Endocrinol Invest (2016) 39:607–15. doi: 10.1007/s40618-015-0408-2 [DOI] [PubMed] [Google Scholar]
- 62. Dionysopoulou S, Charmandari E, Bargiota A, Vlahos N, Mastorakos G, Valsamakis G. The role of hypothalamic inflammation in diet-induced obesity and its association with cognitive and mood disorders. Nutrients (2021) 13(2):498. doi: 10.3390/nu13020498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Rand CM, Patwari PP, Rodikova EA, Zhou L, Berry-Kravis EM, Wilson RJ, et al. Rapid-onset obesity with hypothalamic dysfunction, hypoventilation, and autonomic dysregulation: analysis of hypothalamic and autonomic candidate genes. Pediatr Res (2011) 70:375–8. doi: 10.1203/PDR.0b013e318229474d [DOI] [PubMed] [Google Scholar]
- 64. Agus A, Denizot J, Thevenot J, Martinez-Medina M, Massier S, Sauvanet P, et al. Western Diet induces a shift in microbiota composition enhancing susceptibility to adherent-invasive e. coli infection and intestinal inflammation. Sci Rep (2016) 6:19032. doi: 10.1038/srep19032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Poon K. Behavioral feeding circuit: Dietary fat-induced effects of inflammatory mediators in the hypothalamus. Front Endocrinol (Lausanne) (2020) 11:591559. doi: 10.3389/fendo.2020.591559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Thaler JP, Yi CX, Schur EA, Guyenet SJ, Hwang BH, Dietrich MO, et al. Obesity is associated with hypothalamic injury in rodents and humans. J Clin Invest (2012) 122:153–62. doi: 10.1172/JCI59660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Koch M, Horvath TL. Molecular and cellular regulation of hypothalamic melanocortin neurons controlling food intake and energy metabolism. Mol Psychiatry (2014) 19:752–61. doi: 10.1038/mp.2014.30 [DOI] [PubMed] [Google Scholar]
- 68. Kim JD, Leyva S, Diano S. Hormonal regulation of the hypothalamic melanocortin system. Front Physiol (2014) 5:480. doi: 10.3389/fphys.2014.00480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Kuhnen P, Krude H, Biebermann H. Melanocortin-4 receptor signalling: Importance for weight regulation and obesity treatment. Trends Mol Med (2019) 25:136–48. doi: 10.1016/j.molmed.2018.12.002 [DOI] [PubMed] [Google Scholar]
- 70. Lotta LA, Mokrosinski J, Mendes de Oliveira E, Li C, Sharp SJ, Luan J, et al. Human gain-of-Function MC4R variants show signaling bias and protect against obesity. Cell (2019) 177:597–607 e9. doi: 10.1016/j.cell.2019.03.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Ayers KL, Glicksberg BS, Garfield AS, Longerich S, White JA, Yang P, et al. Melanocortin 4 receptor pathway dysfunction in obesity: Patient stratification aimed at MC4R agonist treatment. J Clin Endocrinol Metab (2018) 103:2601–12. doi: 10.1210/jc.2018-00258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Kim SH, Ahn MB, Cho WK, Cho KS, Jung MH, Suh BK. The relation of serum nesfatin-1 level with anthropometric and metabolic parameters in children and adolescents: A prospective observational study. Med (Baltimore) (2019) 98:e15460. doi: 10.1097/MD.0000000000015460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Alotibi MN, Alnoury AM, Alhozali AM. Serum nesfatin-1 and galanin concentrations in the adult with metabolic syndrome. Relat to insulin resistance Obes Saudi Med J (2019) 40:19–25. doi: 10.15537/smj.2019.1.22825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Ravussin A, Youm YH, Sander J, Ryu S, Nguyen K, Varela L, et al. Loss of nucleobindin-2 causes insulin resistance in obesity without impacting satiety or adiposity. Cell Rep (2018) 24:1085–92.e6. doi: 10.1016/j.celrep.2018.06.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Psilopanagioti A, Nikou S, Papadaki H. Nucleobindin-2/Nesfatin-1 in the human hypothalamus is reduced in obese subjects and colocalizes with oxytocin, vasopressin, melanin-concentrating hormone, and cocaine- and amphetamine-regulated transcript. Neuroendocrinology (2019) 108:190–200. doi: 10.1159/000496731 [DOI] [PubMed] [Google Scholar]
- 76. Yang M, Zhang Z, Wang C, Li K, Li S, Boden G, et al. Nesfatin-1 action in the brain increases insulin sensitivity through Akt/AMPK/TORC2 pathway in diet-induced insulin resistance. Diabetes (2012) 61:1959–68. doi: 10.2337/db11-1755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Ozturk Ozkan G. Effects of nesfatin-1 on food intake and hyperglycemia. J Am Coll Nutr (2020) 39:345–51. doi: 10.1080/07315724.2019.1646678 [DOI] [PubMed] [Google Scholar]
- 78. Zhai T, Li SZ, Fan XT, Tian Z, Lu XQ, Dong J. Circulating nesfatin-1 levels and type 2 diabetes: A systematic review and meta-analysis. J Diabetes Res (2017) 2017:7687098. doi: 10.1155/2017/7687098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Adamska E, Ostrowska L, Gorska M, Kretowski A. The role of gastrointestinal hormones in the pathogenesis of obesity and type 2 diabetes. Prz Gastroenterol (2014) 9:69–76. doi: 10.5114/pg.2014.42498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Gribble FM, Reimann F. Metabolic messengers: glucagon-like peptide 1. Nat Metab (2021) 3:142–8. doi: 10.1038/s42255-020-00327-x [DOI] [PubMed] [Google Scholar]
- 81. Ribeiro-Parenti L, Jarry AC, Cavin JB, Willemetz A, Le Beyec J, Sannier A, et al. Bariatric surgery induces a new gastric mucosa phenotype with increased functional glucagon-like peptide-1 expressing cells. Nat Commun (2021) 12:110. doi: 10.1038/s41467-020-20301-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Roth CL, Perez FA, Whitlock KB, Elfers C, Yanovski JA, Shoemaker AH, et al. A phase 3 randomized clinical trial using a once-weekly glucagon-like peptide-1 receptor agonist in adolescents and young adults with hypothalamic obesity. Diabetes Obes Metab (2021) 23:363–73. doi: 10.1111/dom.14224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Jensterle M, Rizzo M, Janez A. Glucagon-like peptide 1 and taste perception: From molecular mechanisms to potential clinical implications. Int J Mol Sci (2021) 22(2):902. doi: 10.3390/ijms22020902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Cawthon CR, de la Serre CB. The critical role of CCK in the regulation of food intake and diet-induced obesity. Peptides (2021) 138:170492. doi: 10.1016/j.peptides.2020.170492 [DOI] [PubMed] [Google Scholar]
- 85. Lafferty RA, Flatt PR, Irwin N. Emerging therapeutic potential for peptide YY for obesity-diabetes. Peptides (2018) 100:269–74. doi: 10.1016/j.peptides.2017.11.005 [DOI] [PubMed] [Google Scholar]
- 86. Moran TH, Bi S. Hyperphagia and obesity in OLETF rats lacking CCK-1 receptors. Philos Trans R Soc Lond B Biol Sci (2006) 361:1211–8. doi: 10.1098/rstb.2006.1857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Zhang L, Bijker MS, Herzog H. The neuropeptide y system: pathophysiological and therapeutic implications in obesity and cancer. Pharmacol Ther (2011) 131:91–113. doi: 10.1016/j.pharmthera.2011.03.011 [DOI] [PubMed] [Google Scholar]
- 88. Dumont Y, Cadieux A, Pheng LH, Fournier A, St-Pierre S, Quirion R. Peptide YY derivatives as selective neuropeptide y/peptide YY Y1 and Y2 agonists devoided of activity for the Y3 receptor sub-type. Brain Res Mol Brain Res (1994) 26:320–4. doi: 10.1016/0169-328X(94)90105-8 [DOI] [PubMed] [Google Scholar]
- 89. Singer K, Morris DL, Oatmen KE, Wang T, DelProposto J, Mergian T, et al. Neuropeptide y is produced by adipose tissue macrophages and regulates obesity-induced inflammation. PloS One (2013) 8:e57929. doi: 10.1371/journal.pone.0057929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Chatree S, Sitticharoon C, Maikaew P, Uawithya P, Chearskul S. Adipose Y5R mRNA is higher in obese than non-obese humans and is correlated with obesity parameters. Exp Biol Med (Maywood) (2018) 243:786–95. doi: 10.1177/1535370218774889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Dozio E, Ruscica M, Motta M, Magni P. Hypothalamic neuropeptide systems as targets for potential anti-obesity drugs. Mini Rev Med Chem (2007) 7:11–9. doi: 10.2174/138955707779317894 [DOI] [PubMed] [Google Scholar]
- 92. Ailanen L, Vahatalo LH, Salomaki-Myftari H, Makela S, Orpana W, Ruohonen ST, et al. Peripherally administered Y2-receptor antagonist BIIE0246 prevents diet-induced obesity in mice with excess neuropeptide y, but enhances obesity in control mice. Front Pharmacol (2018) 9:319. doi: 10.3389/fphar.2018.00319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Monteleone P, Maj M. Dysfunctions of leptin, ghrelin, BDNF and endocannabinoids in eating disorders: beyond the homeostatic control of food intake. Psychoneuroendocrinology (2013) 38:312–30. doi: 10.1016/j.psyneuen.2012.10.021 [DOI] [PubMed] [Google Scholar]
- 94. Rosicka M, Krsek M, Matoulek M, Jarkovska Z, Marek J, Justova V, et al. Serum ghrelin levels in obese patients: the relationship to serum leptin levels and soluble leptin receptors levels. Physiol Res (2003) 52:61–6. [PubMed] [Google Scholar]
- 95. Daghestani MH, Ozand PT, Al-Himadi AR, Al-Odaib AN. Hormonal levels of leptin, insulin, ghrelin, and neuropeptide y in lean, overweight, and obese Saudi females. Saudi Med J (2007) 28:1191–7. [PubMed] [Google Scholar]
- 96. Rasmussen MH. Obesity, growth hormone and weight loss. Mol Cell Endocrinol (2010) 316:147–53. doi: 10.1016/j.mce.2009.08.017 [DOI] [PubMed] [Google Scholar]
- 97. Huang Z, Lu X, Huang L, Zhang C, Veldhuis JD, Cowley MA, et al. Stimulation of endogenous pulsatile growth hormone secretion by activation of growth hormone secretagogue receptor reduces the fat accumulation and improves the insulin sensitivity in obese mice. FASEB J (2021) 35:e21269. doi: 10.1096/fj.202001924RR [DOI] [PubMed] [Google Scholar]
- 98. Dillard JR, Newsome FA, Kelly AS, Gross AC, Morgan-Daniel J, Adkins LE, et al. The effects of anti-obesity pharmacotherapy interventions on psychosocial factors among adolescents with obesity: a scoping review. Curr Nutr Rep (2021) 10(1):58–70. doi: 10.1007/s13668-021-00351-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Kredel LI, Siegmund B. Adipose-tissue and intestinal inflammation - visceral obesity and creeping fat. Front Immunol (2014) 5:462. doi: 10.3389/fimmu.2014.00462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Karagiannides I, Pothoulakis C. Neuropeptides, mesenteric fat, and intestinal inflammation. Ann N Y Acad Sci (2008) 1144:127–35. doi: 10.1196/annals.1418.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Ellulu MS, Patimah I, Khaza'ai H, Rahmat A, Abed Y. Obesity and inflammation: the linking mechanism and the complications. Arch Med Sci (2017) 13:851–63. doi: 10.5114/aoms.2016.58928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Weidinger C, Ziegler JF, Letizia M, Schmidt F, Siegmund B. Adipokines and their role in intestinal inflammation. Front Immunol (2018) 9:1974. doi: 10.3389/fimmu.2018.01974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Ouchi N, Parker JL, Lugus JJ, Walsh K. Adipokines in inflammation and metabolic disease. Nat Rev Immunol (2011) 11:85–97. doi: 10.1038/nri2921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Saia RS, Ribeiro AB, Giusti H. Cholecystokinin modulates the mucosal inflammatory response and prevents the lipopolysaccharide-induced intestinal epithelial barrier dysfunction. Shock (2020) 53:242–51. doi: 10.1097/SHK.0000000000001355 [DOI] [PubMed] [Google Scholar]
- 105. Zihni C, Mills C, Matter K, Balda MS. Tight junctions: from simple barriers to multifunctional molecular gates. Nat Rev Mol Cell Biol (2016) 17:564–80. doi: 10.1038/nrm.2016.80 [DOI] [PubMed] [Google Scholar]
- 106. Moreno-Navarrete JM, Sabater M, Ortega F, Ricart W, Fernandez-Real JM. Circulating zonulin, a marker of intestinal permeability, is increased in association with obesity-associated insulin resistance. PLoS One (2012) 7:e37160. doi: 10.1371/journal.pone.0037160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Genser L, Aguanno D, Soula HA, Dong L, Trystram L, Assmann K, et al. Increased jejunal permeability in human obesity is revealed by a lipid challenge and is linked to inflammation and type 2 diabetes. J Pathol (2018) 246:217–30. doi: 10.1002/path.5134 [DOI] [PubMed] [Google Scholar]
- 108. Chang CS, Kao CY. Current understanding of the gut microbiota shaping mechanisms. J BioMed Sci (2019) 26:59. doi: 10.1186/s12929-019-0554-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Holmes ZC, Silverman JD, Dressman HK, Wei Z, Dallow EP, Armstrong SC, et al. Short-chain fatty acid production by gut microbiota from children with obesity differs according to prebiotic choice and bacterial community composition. mBio (2020) 11(4):e00914–20. doi: 10.1128/mBio.00914-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Aoun A, Darwish F, Hamod N. The influence of the gut microbiome on obesity in adults and the role of probiotics, prebiotics, and synbiotics for weight loss. Prev Nutr Food Sci (2020) 25:113–23. doi: 10.3746/pnf.2020.25.2.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Amabebe E, Robert FO, Agbalalah T, Orubu ESF. Microbial dysbiosis-induced obesity: role of gut microbiota in homoeostasis of energy metabolism. Br J Nutr (2020) 123:1127–37. doi: 10.1017/S0007114520000380 [DOI] [PubMed] [Google Scholar]
- 112. Arora T, Rudenko O, Egerod KL, Husted AS, Kovatcheva-Datchary P, Akrami R, et al. Microbial fermentation of flaxseed fibers modulates the transcriptome of GPR41-expressing enteroendocrine cells and protects mice against diet-induced obesity. Am J Physiol Endocrinol Metab (2019) 316:E453–63. doi: 10.1152/ajpendo.00391.2018 [DOI] [PubMed] [Google Scholar]
- 113. Gao Z, Yin J, Zhang J, Ward RE, Martin RJ, Lefevre M, et al. Butyrate improves insulin sensitivity and increases energy expenditure in mice. Diabetes (2009) 58:1509–17. doi: 10.2337/db08-1637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Vinolo MA, Rodrigues HG, Festuccia WT, Crisma AR, Alves VS, Martins AR, et al. Tributyrin attenuates obesity-associated inflammation and insulin resistance in high-fat-fed mice. Am J Physiol Endocrinol Metab (2012) 303:E272–82. doi: 10.1152/ajpendo.00053.2012 [DOI] [PubMed] [Google Scholar]
- 115. Silva YP, Bernardi A, Frozza RL. The role of short-chain fatty acids from gut microbiota in gut-brain communication. Front Endocrinol (Lausanne) (2020) 11:25. doi: 10.3389/fendo.2020.00025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Heiss CN, Manneras-Holm L, Lee YS, Serrano-Lobo J, Hakansson Gladh A, Seeley RJ, et al. The gut microbiota regulates hypothalamic inflammation and leptin sensitivity in Western diet-fed mice via a GLP-1R-dependent mechanism. Cell Rep (2021) 35:109163. doi: 10.1016/j.celrep.2021.109163 [DOI] [PubMed] [Google Scholar]
- 117. Sheykhsaran E, Abbasi A, Ebrahimzadeh Leylabadlo H, Sadeghi J, Mehri S, Naeimi Mazraeh F, et al. Gut microbiota and obesity: an overview of microbiota to microbial-based therapies. Postgrad Med J (2022) 2021:141311. doi: 10.1136/postgradmedj-2021-141311 [DOI] [PubMed] [Google Scholar]
- 118. Ngowi EE, Wang YZ, Khattak S, Khan NH, Sayed Mohamed Mahmoud S, Helmy Y, et al. Impact of the factors shaping gut microbiota on obesity. J Appl Microbiol (2021) 31(5):2131–47. doi: 10.1111/jam.15036 [DOI] [PubMed] [Google Scholar]
- 119. Maier TV, Lucio M, Lee LH, VerBerkmoes NC, Brislawn CJ, Bernhardt J, et al. Impact of dietary resistant starch on the human gut microbiome, metaproteome, and metabolome. mBio (2017) 8(5):e01343–17. doi: 10.1128/mBio.01343-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Chelakkot C, Choi Y, Kim DK, Park HT, Ghim J, Kwon Y, et al. Akkermansia muciniphila-derived extracellular vesicles influence gut permeability through the regulation of tight junctions. Exp Mol Med (2018) 50:e450. doi: 10.1038/emm.2017.282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Xu Y, Wang N, Tan HY, Li S, Zhang C, Feng Y. Function of akkermansia muciniphila in obesity: Interactions with lipid metabolism, immune response and gut systems. Front Microbiol (2020) 11:219. doi: 10.3389/fmicb.2020.00219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Sugama J, Moritoh Y, Yashiro H, Tsuchimori K, Watanabe M. Enteropeptidase inhibition improves obesity by modulating gut microbiota composition and enterobacterial metabolites in diet-induced obese mice. Pharmacol Res (2021) 163:105337. doi: 10.1016/j.phrs.2020.105337 [DOI] [PubMed] [Google Scholar]
- 123. Christensen L, Vuholm S, Roager HM, Nielsen DS, Krych L, Kristensen M, et al. Prevotella abundance predicts weight loss success in healthy, overweight adults consuming a whole-grain diet ad libitum: A Post hoc analysis of a 6-wk randomized controlled trial. J Nutr (2019) 149:2174–81. doi: 10.1093/jn/nxz198 [DOI] [PubMed] [Google Scholar]
- 124. Zawada A, Rychter AM, Ratajczak AE, Lisiecka-Masian A, Dobrowolska A, Krela-Kazmierczak I. Does gut-microbiome interaction protect against obesity and obesity-associated metabolic disorders? Microorganisms (2020) 9(1):18. doi: 10.3390/microorganisms9010018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Kuhne SG, Stengel A. Alteration of peptidergic gut-brain signaling under conditions of obesity. J Physiol Pharmacol (2019) 70(5). doi: 10.26402/jpp.2019.5.01 [DOI] [PubMed] [Google Scholar]
- 126. Khan S, Luck H, Winer S, Winer DA. Emerging concepts in intestinal immune control of obesity-related metabolic disease. Nat Commun (2021) 12:2598. doi: 10.1038/s41467-021-22727-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Monteiro-Sepulveda M, Touch S, Mendes-Sa C, Andre S, Poitou C, Allatif O, et al. Jejunal T cell inflammation in human obesity correlates with decreased enterocyte insulin signaling. Cell Metab (2015) 22:113–24. doi: 10.1016/j.cmet.2015.05.020 [DOI] [PubMed] [Google Scholar]
- 128. Wensveen FM, Valentic S, Sestan M, Wensveen TT, Polic B. Interactions between adipose tissue and the immune system in health and malnutrition. Semin Immunol (2015) 27:322–33. doi: 10.1016/j.smim.2015.10.006 [DOI] [PubMed] [Google Scholar]
- 129. Fernandez-Riejos P, Najib S, Santos-Alvarez J, Martin-Romero C, Perez-Perez A, Gonzalez-Yanes C, et al. Role of leptin in the activation of immune cells. Mediators Inflammation (2010) 2010:568343. doi: 10.1155/2010/568343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Tabata M, Kadomatsu T, Fukuhara S, Miyata K, Ito Y, Endo M, et al. Angiopoietin-like protein 2 promotes chronic adipose tissue inflammation and obesity-related systemic insulin resistance. Cell Metab (2009) 10:178–88. doi: 10.1016/j.cmet.2009.08.003 [DOI] [PubMed] [Google Scholar]
- 131. Benomar Y, Gertler A, De Lacy P, Crepin D, Ould Hamouda H, Riffault L, et al. Central resistin overexposure induces insulin resistance through toll-like receptor 4. Diabetes (2013) 62:102–14. doi: 10.2337/db12-0237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Cottam MA, Caslin HL, Winn NC, Hasty AH. Multiomics reveals persistence of obesity-associated immune cell phenotypes in adipose tissue during weight loss and weight regain in mice. Nat Commun (2022) 13:2950. doi: 10.1038/s41467-022-30646-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Liu R, Nikolajczyk BS. Tissue immune cells fuel obesity-associated inflammation in adipose tissue and beyond. Front Immunol (2019) 10:1587. doi: 10.3389/fimmu.2019.01587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Hong CP, Park A, Yang BG, Yun CH, Kwak MJ, Lee GW, et al. Gut-specific delivery of T-helper 17 cells reduces obesity and insulin resistance in mice. Gastroenterology (2017) 152:1998–2010. doi: 10.1053/j.gastro.2017.02.016 [DOI] [PubMed] [Google Scholar]
- 135. Luck H, Tsai S, Chung J, Clemente-Casares X, Ghazarian M, Revelo XS, et al. Regulation of obesity-related insulin resistance with gut anti-inflammatory agents. Cell Metab (2015) 21:527–42. doi: 10.1016/j.cmet.2015.03.001 [DOI] [PubMed] [Google Scholar]
- 136. Arpaia N, Campbell C, Fan X, Dikiy S, van der Veeken J, deRoos P, et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature (2013) 504:451–5. doi: 10.1038/nature12726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Shin NR, Lee JC, Lee HY, Kim MS, Whon TW, Lee MS, et al. An increase in the akkermansia spp. population induced by metformin treatment improves glucose homeostasis in diet-induced obese mice. Gut (2014) 63:727–35. doi: 10.1136/gutjnl-2012-303839 [DOI] [PubMed] [Google Scholar]
- 138. Moya-Perez A, Neef A, Sanz Y. Bifidobacterium pseudocatenulatum CECT 7765 reduces obesity-associated inflammation by restoring the lymphocyte-macrophage balance and gut microbiota structure in high-fat diet-fed mice. PLoS One (2015) 10:e0126976. doi: 10.1371/journal.pone.0126976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Gauffin Cano P, Santacruz A, Moya A, Sanz Y. Bacteroides uniformis CECT 7771 ameliorates metabolic and immunological dysfunction in mice with high-fat-diet induced obesity. PLoS One (2012) 7:e41079. doi: 10.1371/journal.pone.0041079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Scheithauer TPM, Rampanelli E, Nieuwdorp M, Vallance BA, Verchere CB, van Raalte DH, et al. Gut microbiota as a trigger for metabolic inflammation in obesity and type 2 diabetes. Front Immunol (2020) 11:571731. doi: 10.3389/fimmu.2020.571731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Everard A, Belzer C, Geurts L, Ouwerkerk JP, Druart C, Bindels LB, et al. Cross-talk between akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc Natl Acad Sci U.S.A. (2013) 110:9066–71. doi: 10.1073/pnas.1219451110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Forte N, Fernandez-Rilo AC, Palomba L, Di Marzo V, Cristino L. Obesity affects the microbiota-Gut-Brain axis and the regulation thereof by endocannabinoids and related mediators. Int J Mol Sci (2020) 21(5):1554. doi: 10.3390/ijms21051554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Rockl KS, Witczak CA, Goodyear LJ. Signaling mechanisms in skeletal muscle: acute responses and chronic adaptations to exercise. IUBMB Life (2008) 60:145–53. doi: 10.1002/iub.21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Costa RJS, Snipe RMJ, Kitic CM, Gibson PR. Systematic review: exercise-induced gastrointestinal syndrome-implications for health and intestinal disease. Aliment Pharmacol Ther (2017) 46:246–65. doi: 10.1111/apt.14157 [DOI] [PubMed] [Google Scholar]
- 145. Douglas JA, Deighton K, Atkinson JM, Sari-Sarraf V, Stensel DJ, Atkinson G. Acute exercise and appetite-regulating hormones in overweight and obese individuals: A meta-analysis. J Obes (2016) 2016:2643625. doi: 10.1155/2016/2643625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Broom DR, Stensel DJ, Bishop NC, Burns SF, Miyashita M. Exercise-induced suppression of acylated ghrelin in humans. J Appl Physiol (1985. 2007) 102:2165–71. doi: 10.1152/japplphysiol.00759.2006 [DOI] [PubMed] [Google Scholar]
- 147. Broom DR, Batterham RL, King JA, Stensel DJ. Influence of resistance and aerobic exercise on hunger, circulating levels of acylated ghrelin, and peptide YY in healthy males. Am J Physiol Regul Integr Comp Physiol (2009) 296:R29–35. doi: 10.1152/ajpregu.90706.2008 [DOI] [PubMed] [Google Scholar]
- 148. Ueda SY, Yoshikawa T, Katsura Y, Usui T, Nakao H, Fujimoto S. Changes in gut hormone levels and negative energy balance during aerobic exercise in obese young males. J Endocrinol (2009) 201:151–9. doi: 10.1677/JOE-08-0500 [DOI] [PubMed] [Google Scholar]
- 149. King JA, Miyashita M, Wasse LK, Stensel DJ. Influence of prolonged treadmill running on appetite, energy intake and circulating concentrations of acylated ghrelin. Appetite (2010) 54:492–8. doi: 10.1016/j.appet.2010.02.002 [DOI] [PubMed] [Google Scholar]
- 150. King JA, Wasse LK, Broom DR, Stensel DJ. Influence of brisk walking on appetite, energy intake, and plasma acylated ghrelin. Med Sci Sports Exerc (2010) 42:485–92. doi: 10.1249/MSS.0b013e3181ba10c4 [DOI] [PubMed] [Google Scholar]
- 151. Becker GF, Macedo RC, Cunha Gdos S, Martins JB, Laitano O, Reischak-Oliveira A. Combined effects of aerobic exercise and high-carbohydrate meal on plasma acylated ghrelin and levels of hunger. Appl Physiol Nutr Metab (2012) 37:184–92. doi: 10.1139/h11-149 [DOI] [PubMed] [Google Scholar]
- 152. Deighton K, Barry R, Connon CE, Stensel DJ. Appetite, gut hormone and energy intake responses to low volume sprint interval and traditional endurance exercise. Eur J Appl Physiol (2013) 113:1147–56. doi: 10.1007/s00421-012-2535-1 [DOI] [PubMed] [Google Scholar]
- 153. King JA, Wasse LK, Stensel DJ. The acute effects of swimming on appetite, food intake, and plasma acylated ghrelin. J Obes 2011 (2011) 2011:351628. doi: 10.1155/2011/351628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Vanderheyden LW, McKie GL, Howe GJ, Hazell TJ. Greater lactate accumulation following an acute bout of high-intensity exercise in males suppresses acylated ghrelin and appetite postexercise. J Appl Physiol (1985. 2020) 128:1321–8. doi: 10.1152/japplphysiol.00081.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Hallworth JR, Copeland JL, Doan J, Hazell TJ. The effect of exercise intensity on total PYY and GLP-1 in healthy females: A pilot study. J Nutr Metab (2017) 2017:4823102. doi: 10.1155/2017/4823102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Balaguera-Cortes L, Wallman KE, Fairchild TJ, Guelfi KJ. Energy intake and appetite-related hormones following acute aerobic and resistance exercise. Appl Physiol Nutr Metab (2011) 36:958–66. doi: 10.1139/h11-121 [DOI] [PubMed] [Google Scholar]
- 157. Okada TE, Quan T, Bomhof MR. Exogenous ketones lower post-exercise acyl-ghrelin and GLP-1 but do not impact ad libitum energy intake. Front Nutr (2020) 7:626480. doi: 10.3389/fnut.2020.626480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Hazell TJ, Islam H, Townsend LK, Schmale MS, Copeland JL. Effects of exercise intensity on plasma concentrations of appetite-regulating hormones: Potential mechanisms. Appetite (2016) 98:80–8. doi: 10.1016/j.appet.2015.12.016 [DOI] [PubMed] [Google Scholar]
- 159. Martins C, Kulseng B, Rehfeld JF, King NA, Blundell JE. Effect of chronic exercise on appetite control in overweight and obese individuals. Med Sci Sports Exerc (2013) 45:805–12. doi: 10.1249/MSS.0b013e31827d1618 [DOI] [PubMed] [Google Scholar]
- 160. Kanaley JA, Heden TD, Liu Y, Whaley-Connell AT, Chockalingam A, Dellsperger KC, et al. Short-term aerobic exercise training increases postprandial pancreatic polypeptide but not peptide YY concentrations in obese individuals. Int J Obes (Lond) (2014) 38:266–71. doi: 10.1038/ijo.2013.84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Wynne K, Park AJ, Small CJ, Meeran K, Ghatei MA, Frost GS, et al. Oxyntomodulin increases energy expenditure in addition to decreasing energy intake in overweight and obese humans: a randomised controlled trial. Int J Obes (Lond) (2006) 30:1729–36. doi: 10.1038/sj.ijo.0803344 [DOI] [PubMed] [Google Scholar]
- 162. Solomon TP, Haus JM, Kelly KR, Cook MD, Filion J, Rocco M, et al. A low-glycemic index diet combined with exercise reduces insulin resistance, postprandial hyperinsulinemia, and glucose-dependent insulinotropic polypeptide responses in obese, prediabetic humans. Am J Clin Nutr (2010) 92:1359–68. doi: 10.3945/ajcn.2010.29771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Shaodong C, Haihong Z, Manting L, Guohui L, Zhengxiao Z, Zhang MY. Research of influence and mechanism of combining exercise with diet control on a model of lipid metabolism rat induced by high fat diet. Lipids Health Dis (2013) 12:21. doi: 10.1186/1476-511X-12-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Ackel-D'Elia C, Carnier J, Bueno CR, Jr., Campos RM, Sanches PL, Clemente AP, et al. Effects of different physical exercises on leptin concentration in obese adolescents. Int J Sports Med (2014) 35:164–71. doi: 10.1055/s-0033-1345128 [DOI] [PubMed] [Google Scholar]
- 165. Markofski MM, Carrillo AE, Timmerman KL, Jennings K, Coen PM, Pence BD, et al. Exercise training modifies ghrelin and adiponectin concentrations and is related to inflammation in older adults. J Gerontol A Biol Sci Med Sci (2014) 69:675–81. doi: 10.1093/gerona/glt132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Ding C, Chooi YUC, Chan Z, Lo J, Choo J, Ding BTK, et al. Dose-dependent effects of exercise and diet on insulin sensitivity and secretion. Med Sci Sports Exerc (2019) 51:2109–16. doi: 10.1249/MSS.0000000000002020 [DOI] [PubMed] [Google Scholar]
- 167. Ramson R, Jurimae J, Jurimae T, Maestu J. The effect of 4-week training period on plasma neuropeptide y, leptin and ghrelin responses in male rowers. Eur J Appl Physiol (2012) 112:1873–80. doi: 10.1007/s00421-011-2166-y [DOI] [PubMed] [Google Scholar]
- 168. Henagan TM, Phillips MD, Cheek DJ, Kirk KM, Barbee JJ, Stewart LK. The melanocortin 3 receptor: a novel mediator of exercise-induced inflammation reduction in postmenopausal women? J Aging Res (2011) 2011:512593. doi: 10.4061/2011/512593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Kraemer RR, Acevedo EO, Synovitz LB, Durand RJ, Johnson LG, Petrella E, et al. Glucoregulatory endocrine responses to intermittent exercise of different intensities: plasma changes in a pancreatic beta-cell peptide, amylin. Metabolism (2002) 51:657–63. doi: 10.1053/meta.2002.32023 [DOI] [PubMed] [Google Scholar]
- 170. Messina G, Di Bernardo G, Viggiano A, De Luca V, Monda V, Messina A, et al. Exercise increases the level of plasma orexin a in humans. J Basic Clin Physiol Pharmacol (2016) 27:611–6. doi: 10.1515/jbcpp-2015-0133 [DOI] [PubMed] [Google Scholar]
- 171. Choi KM, Kim JH, Cho GJ, Baik SH, Park HS, Kim SM. Effect of exercise training on plasma visfatin and eotaxin levels. Eur J Endocrinol (2007) 157:437–42. doi: 10.1530/EJE-07-0127 [DOI] [PubMed] [Google Scholar]
- 172. Ghanbari-Niaki A, Saghebjoo M, Soltani R, Kirwan JP. Plasma visfatin is increased after high-intensity exercise. Ann Nutr Metab (2010) 57:3–8. doi: 10.1159/000313936 [DOI] [PubMed] [Google Scholar]
- 173. Amanat S, Sinaei E, Panji M, MohammadporHodki R, Bagheri-Hosseinabadi Z, Asadimehr H, et al. A randomized controlled trial on the effects of 12 weeks of aerobic, resistance, and combined exercises training on the serum levels of nesfatin-1, irisin-1 and HOMA-IR. Front Physiol (2020) 11:562895. doi: 10.3389/fphys.2020.562895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Mani BK, Castorena CM, Osborne-Lawrence S, Vijayaraghavan P, Metzger NP, Elmquist JK, et al. Ghrelin mediates exercise endurance and the feeding response post-exercise. Mol Metab (2018) 9:114–30. doi: 10.1016/j.molmet.2018.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Algul S, Ozdenk C, Ozcelik O. Variations in leptin, nesfatin-1 and irisin levels induced by aerobic exercise in young trained and untrained male subjects. Biol Sport (2017) 34:339–44. doi: 10.5114/biolsport.2017.69821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Ozcelik O, Algul S, Yilmaz B. Nesfatin-1 and irisin levels in response to the soccer matches performed in morning, afternoon and at night in young trained male subjects. Cell Mol Biol (Noisy-le-grand) (2018) 64:130–3. doi: 10.14715/cmb/2018.64.10.21 [DOI] [PubMed] [Google Scholar]
- 177. Oliveira CLP, Boule NG, Berg A, Sharma AM, Elliott SA, Siervo M, et al. Consumption of a high-protein meal replacement leads to higher fat oxidation, suppression of hunger, and improved metabolic profile after an exercise session. Nutrients (2021) 13(1):155. doi: 10.3390/nu13010155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Tolentino M, Palheta-Junior RC, Silva CMS, Cavalcante AKM, Quetz JDS, Havt A, et al. Role of cholecystokinin and oxytocin in slower gastric emptying induced by physical exercise in rats. Physiol Behav (2021) 233:113355. doi: 10.1016/j.physbeh.2021.113355 [DOI] [PubMed] [Google Scholar]
- 179. Guelfi KJ, Donges CE, Duffield R. Beneficial effects of 12 weeks of aerobic compared with resistance exercise training on perceived appetite in previously sedentary overweight and obese men. Metabolism (2013) 62:235–43. doi: 10.1016/j.metabol.2012.08.002 [DOI] [PubMed] [Google Scholar]
- 180. Shakiba E, Sheikholeslami-Vatani D, Rostamzadeh N, Karim H. The type of training program affects appetite-regulating hormones and body weight in overweight sedentary men. Appl Physiol Nutr Metab (2019) 44:282–7. doi: 10.1139/apnm-2018-0197 [DOI] [PubMed] [Google Scholar]
- 181. Gomes JR, Freitas JR, Grassiolli S. Effects of physical exercise on the intestinal mucosa of rats submitted to a hypothalamic obesity condition. Anat Rec (Hoboken) (2016) 299:1389–96. doi: 10.1002/ar.23453 [DOI] [PubMed] [Google Scholar]
- 182. Zuhl M, Schneider S, Lanphere K, Conn C, Dokladny K, Moseley P. Exercise regulation of intestinal tight junction proteins. Br J Sports Med (2014) 48:980–6. doi: 10.1136/bjsports-2012-091585 [DOI] [PubMed] [Google Scholar]
- 183. Shin HE, Kwak SE, Zhang DD, Lee J, Yoon KJ, Cho HS, et al. Effects of treadmill exercise on the regulation of tight junction proteins in aged mice. Exp Gerontol (2020) 141:111077. doi: 10.1016/j.exger.2020.111077 [DOI] [PubMed] [Google Scholar]
- 184. Matsumoto M, Inoue R, Tsukahara T, Ushida K, Chiji H, Matsubara N, et al. Voluntary running exercise alters microbiota composition and increases n-butyrate concentration in the rat cecum. Biosci Biotechnol Biochem (2008) 72:572–6. doi: 10.1271/bbb.70474 [DOI] [PubMed] [Google Scholar]
- 185. Estaki M, Pither J, Baumeister P, Little JP, Gill SK, Ghosh S, et al. Cardiorespiratory fitness as a predictor of intestinal microbial diversity and distinct metagenomic functions. Microbiome (2016) 4:42. doi: 10.1186/s40168-016-0189-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Ortiz-Alvarez L, Xu H, Martinez-Tellez B. Influence of exercise on the human gut microbiota of healthy adults: A systematic review. Clin Transl Gastroenterol (2020) 11:e00126. doi: 10.14309/ctg.0000000000000126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Quiroga R, Nistal E, Estebanez B, Porras D, Juarez-Fernandez M, Martinez-Florez S, et al. Exercise training modulates the gut microbiota profile and impairs inflammatory signaling pathways in obese children. Exp Mol Med (2020) 52:1048–61. doi: 10.1038/s12276-020-0459-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Ticinesi A, Lauretani F, Tana C, Nouvenne A, Ridolo E, Meschi T. Exercise and immune system as modulators of intestinal microbiome: implications for the gut-muscle axis hypothesis. Exerc Immunol Rev (2019) 25:84–95. [PubMed] [Google Scholar]
- 189. de Sire R, Rizzatti G, Ingravalle F, Pizzoferrato M, Petito V, Lopetuso L, et al. Skeletal muscle-gut axis: emerging mechanisms of sarcopenia for intestinal and extra intestinal diseases. Minerva Gastroenterol Dietol (2018) 64:351–62. doi: 10.23736/S1121-421X.18.02511-4 [DOI] [PubMed] [Google Scholar]
- 190. Laurens C, Bergouignan A, Moro C. Exercise-released myokines in the control of energy metabolism. Front Physiol (2020) 11:91. doi: 10.3389/fphys.2020.00091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Severinsen MCK, Pedersen BK. Muscle-organ crosstalk: The emerging roles of myokines. Endocr Rev (2020) 41(4):594–609. doi: 10.1210/endrev/bnaa016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Bilski J, Mazur-Bialy AI, Surmiak M, Hubalewska-Mazgaj M, Pokorski J, Nitecki J, et al. Effect of acute sprint exercise on myokines and food intake hormones in young healthy men. Int J Mol Sci (2020) 21(22):8848. doi: 10.3390/ijms21228848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Suriano F, Van Hul M, Cani PD. Gut microbiota and regulation of myokine-adipokine function. Curr Opin Pharmacol (2020) 52:9–17. doi: 10.1016/j.coph.2020.03.006 [DOI] [PubMed] [Google Scholar]
- 194. Goh J, Niksirat N, Campbell KL. Exercise training and immune crosstalk in breast cancer microenvironment: exploring the paradigms of exercise-induced immune modulation and exercise-induced myokines. Am J Transl Res (2014) 6:422–38. [PMC free article] [PubMed] [Google Scholar]
- 195. Ahn N, Kim K. Effects of aerobic and resistance exercise on myokines in high fat diet-induced middle-aged obese rats. Int J Environ Res Public Health (2020) 17(8):2685. doi: 10.3390/ijerph17082685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Lucas S, Taront S, Magnan C, Fauconnier L, Delacre M, Macia L, et al. Interleukin-7 regulates adipose tissue mass and insulin sensitivity in high-fat diet-fed mice through lymphocyte-dependent and independent mechanisms. PLoS One (2012) 7:e40351. doi: 10.1371/journal.pone.0040351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Sun H, Ma Y, Gao M, Liu D. IL-15/sIL-15Ralpha gene transfer induces weight loss and improves glucose homeostasis in obese mice. Gene Ther (2016) 23:349–56. doi: 10.1038/gt.2016.4 [DOI] [PubMed] [Google Scholar]
- 198. Duan Y, Li F, Wang W, Guo Q, Wen C, Li Y, et al. Interleukin-15 in obesity and metabolic dysfunction: current understanding and future perspectives. Obes Rev (2017) 18:1147–58. doi: 10.1111/obr.12567 [DOI] [PubMed] [Google Scholar]
- 199. Yang H, Chang J, Chen W, Zhao L, Qu B, Tang C, et al. Treadmill exercise promotes interleukin 15 expression in skeletal muscle and interleukin 15 receptor alpha expression in adipose tissue of high-fat diet rats. Endocrine (2013) 43:579–85. doi: 10.1007/s12020-012-9809-6 [DOI] [PubMed] [Google Scholar]
- 200. Muller TD, Bluher M, Tschop MH, DiMarchi RD. Anti-obesity drug discovery: advances and challenges. Nat Rev Drug Discovery (2022) 21:201–23. doi: 10.1038/s41573-021-00337-8 [DOI] [PMC free article] [PubMed] [Google Scholar]