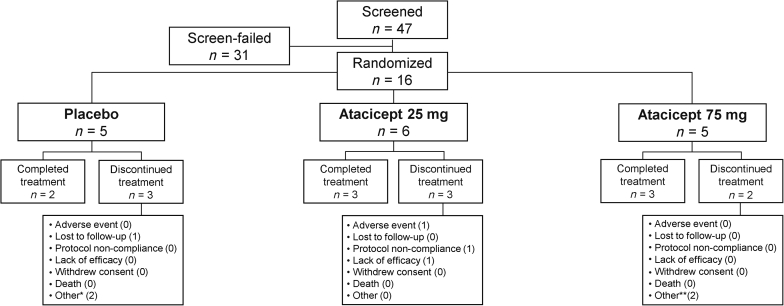
Figure 1.
Patient disposition in the JANUS study. Of the 8 patients who discontinued treatment, 5 completed the safety follow-up period of 24 weeks (placebo, n = 2; atacicept 25 mg, n = 2; atacicept 75 mg, n = 1) and 1 patient completed a safety follow-up period of 12 weeks (placebo group). ∗Both due to study termination by sponsor. ∗∗One due to study termination by sponsor, one due to withdrawal from the study.