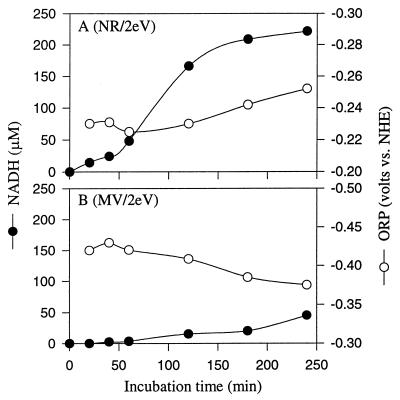
FIG. 4.
Comparison of the effect of electrically reduced NR (A) to that of MV (B) on the chemical reduction of NAD. The experiments were performed in ECB system I. The cathode contained 50 mM Tris-HCl (pH 7.5), 1 mM NAD+, and 100 μM dye. The anode contained 100 mM KPO4 buffer (pH 7.2) and 100 mM NaCl as the electrolyte. The potential was 2.0 V, and the current was 0.8 to 2.4 mA. ORP is an indicator for monitoring how the reactions are going. Decreasing ORP means that the reduction reaction is going very well, but increasing ORP means that the reaction is being disrupted, or the reduced products may be reoxidized.