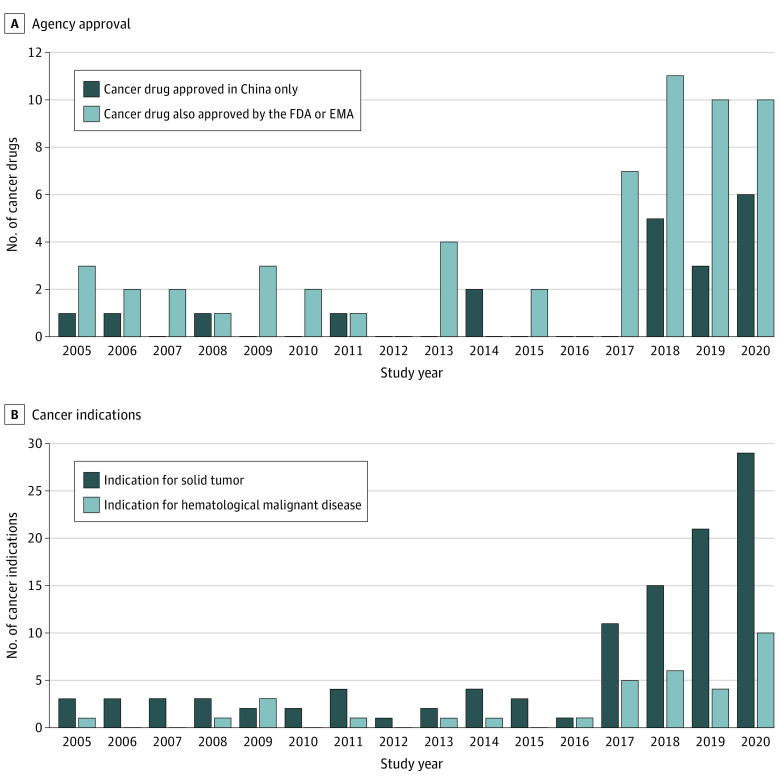
Figure 2. First Marketing Authorization and Supplemental Indication Approval Time of Cancer Drugs Authorized in China Between 2005 and 2020.
A, Cancer drugs approved in China only and cancer drugs also approved by the US Food and Drug Administration (FDA) or the European Medicines Agency (EMA) by December 31, 2020. B, Indications for solid tumors or hematological malignant neoplasms.