FIGURE 4.
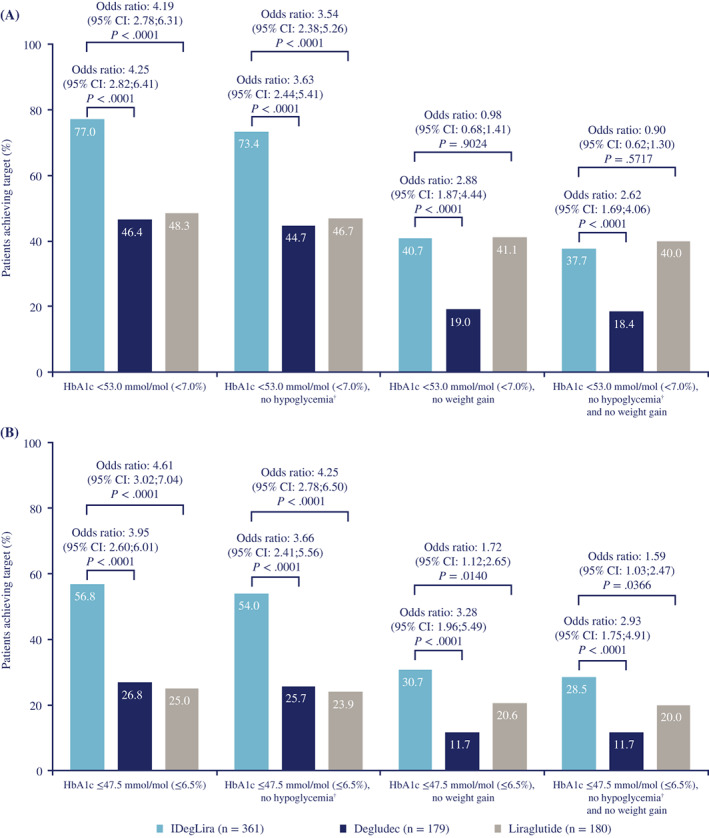
Responder endpoints for (A) HbA1c <53.0 mmoL/moL, and (B) HbA1c ≤48.0 mmoL/moL, and composite endpoints for reaching these targets without weight gain and/or without treatment‐emergent severe or confirmed hypoglycemic episodes† (full analysis set)
†Treatment‐emergent severe or BG‐confirmed hypoglycemic episodes during the final 12 weeks of treatment. Percentages are observed data. Missing values were imputed using last observation carried forward. Analysis after 26 weeks of treatment based on a logistic regression model with treatment and previous oral antidiabetic treatment as fixed factors. The covariate for: ‘Responder for HbA1c <7.0% and for HbA1c ≤6.5%’, and for ‘HbA1c responder endpoints without hypoglycemic episodes’ was baseline HbA1c; covariates for: ‘HbA1c responder endpoints without weight gain’, and for ‘HbA1c responder endpoints without hypoglycemic episodes and weight gain’ were baseline HbA1c and body weight.
BG, blood glucose; CI, confidence interval; degludec, insulin degludec; IDegLira, insulin degludec/liraglutide.
