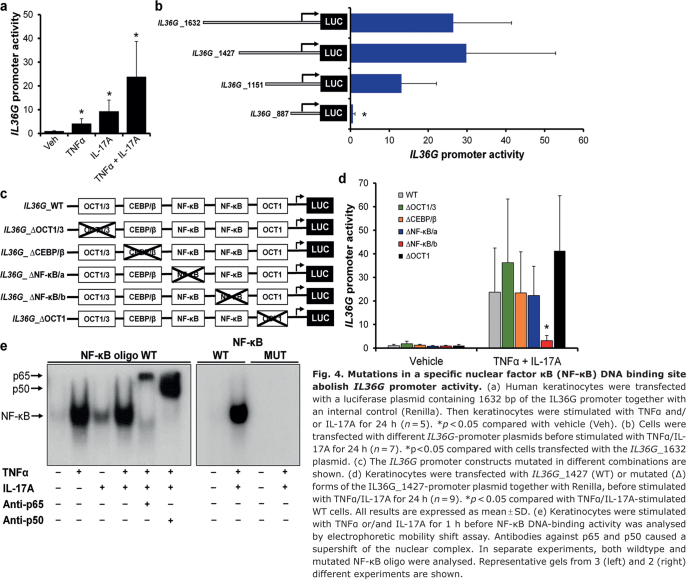
Fig. 4.
Mutations in a specific nuclear factor κB (NF-κB) DNA binding site abolish IL36G promoter activity. (a) Human keratinocytes were transfected with a luciferase plasmid containing 1632 bp of the IL36G promoter together with an internal control (Renilla). Then keratinocytes were stimulated with TNFα and/or IL-17A for 24 h (n = 5). *p < 0.05 compared with vehicle (Veh). (b) Cells were transfected with different IL36G-promoter plasmids before stimulated with TNFα/IL-17A for 24 h (n = 7). *p<0.05 compared with cells transfected with the IL36G_1632 plasmid. (c) The IL36G promoter constructs mutated in different combinations are shown. (d) Keratinocytes were transfected with IL36G_1427 (WT) or mutated (Δ) forms of the IL36G_1427-promoter plasmid together with Renilla, before stimulated with TNFα/IL-17A for 24 h (n = 9). *p < 0.05 compared with TNFα/IL-17A-stimulated WT cells. All results are expressed as mean ± SD. (e) Keratinocytes were stimulated with TNFα or/and IL-17A for 1 h before NF-κB DNA-binding activity was analysed by electrophoretic mobility shift assay. Antibodies against p65 and p50 caused a supershift of the nuclear complex. In separate experiments, both wildtype and mutated NF-κB oligo were analysed. Representative gels from 3 (left) and 2 (right) different experiments are shown.