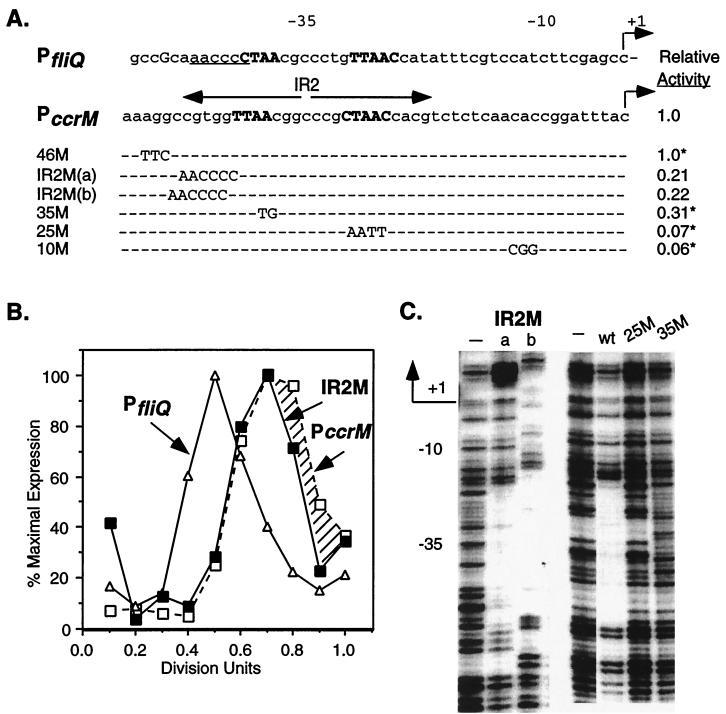
FIG. 6.
Role of the IR2 in regulating PccrM expression. (A) Effects of mutations on ccrM promoter activity. The wild-type fliQ and ccrM promoters are shown; nucleotides in the CtrA recognition sequence are capitalized and boldfaced. Mutant bases in PccrM are shown beneath the wild-type bases being replaced. Promoters with changes in the 5′ region of the inverted repeat [IR2M(a) and IR2M(b)] were constructed by replacing the first 6 nucleotides in IR2 with the bases at these positions in PfliQ (underlined in the wild-type sequence). The IR2M mutations were cloned into pRKlac290 to create transcriptional fusions, and the mutants were assayed for β-galactosidase activity. The activity of control plasmid pCS145, defined here as 1.0, was 3,200 Miller units. Shown for comparison are PccrM mutants and their relative β-galactosidase activities (asterisks) from a previous study (27). (B) Temporal expression of wild-type PfliQ (▵), IR2M (■), and wild-type PccrM (□) in synchronous LS2531 cultures. See the legend to Fig. 1 for details. The hatched region indicates the differences in the expression of the wild-type and mutant ccrM promoters. (C) DNase I protection of ccrM mutant promoters and the wild-type ccrM promoter (wt) by His6-CtrA∼P (50 μg/ml). Data for the IR2M(a) and IR2M(b) mutant promoters and promoters with changes at conserved bases −25 to −28 (25M) and bases −35 and −36 (35M) are shown. The regions protected by CtrA∼P in the wild-type and IR2M promoters are of the same size. The protected regions in the IR2M(a) and IR2M(b) promoters are at different positions because the 5′ end of the IR2M(b) template contained an additional 3 nucleotides. Control reactions without His6-CtrA∼P are indicated by minus signs.