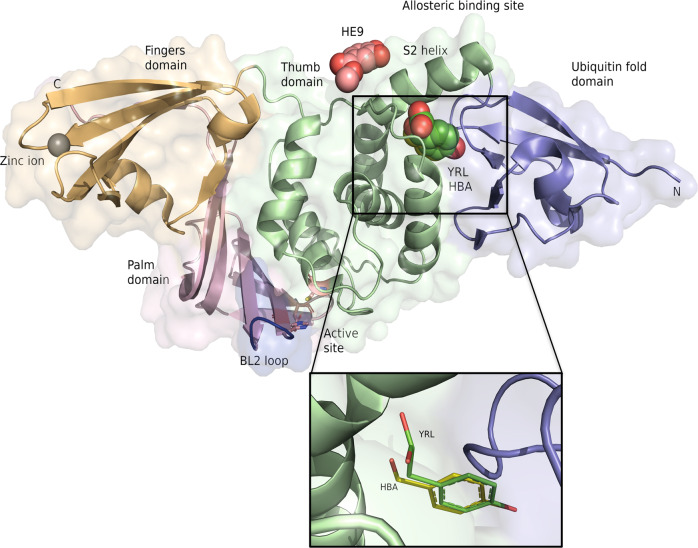
Fig. 1. Crystal structures of SARS-CoV-2 PLpro complexes with the three natural compounds.
PLpro domains are depicted in a right-handed architecture, ubiquitin-fold like (blue), thumb (green), palm (salmon pink), and fingers (light orange). Catalytic active site residues Cys 111, His 272, and Asp 286 are represented as sticks and a zinc ion in the fingers domain is shown as a gray sphere. The flexible blocking loop (BL2 loop) that changes conformation in the context of substrate binding is shown in blue. YRL (green spheres), HBA (yellow spheres) and HE9 (pink spheres) compounds bind at the allosteric site that is located about 30 Å apart to the active site. S2 helix involved in the interaction of the ISG15 molecule is indicated. The inset shows an enlarged view of the two compounds HBA and YRL in the binding site.