Figure 1.
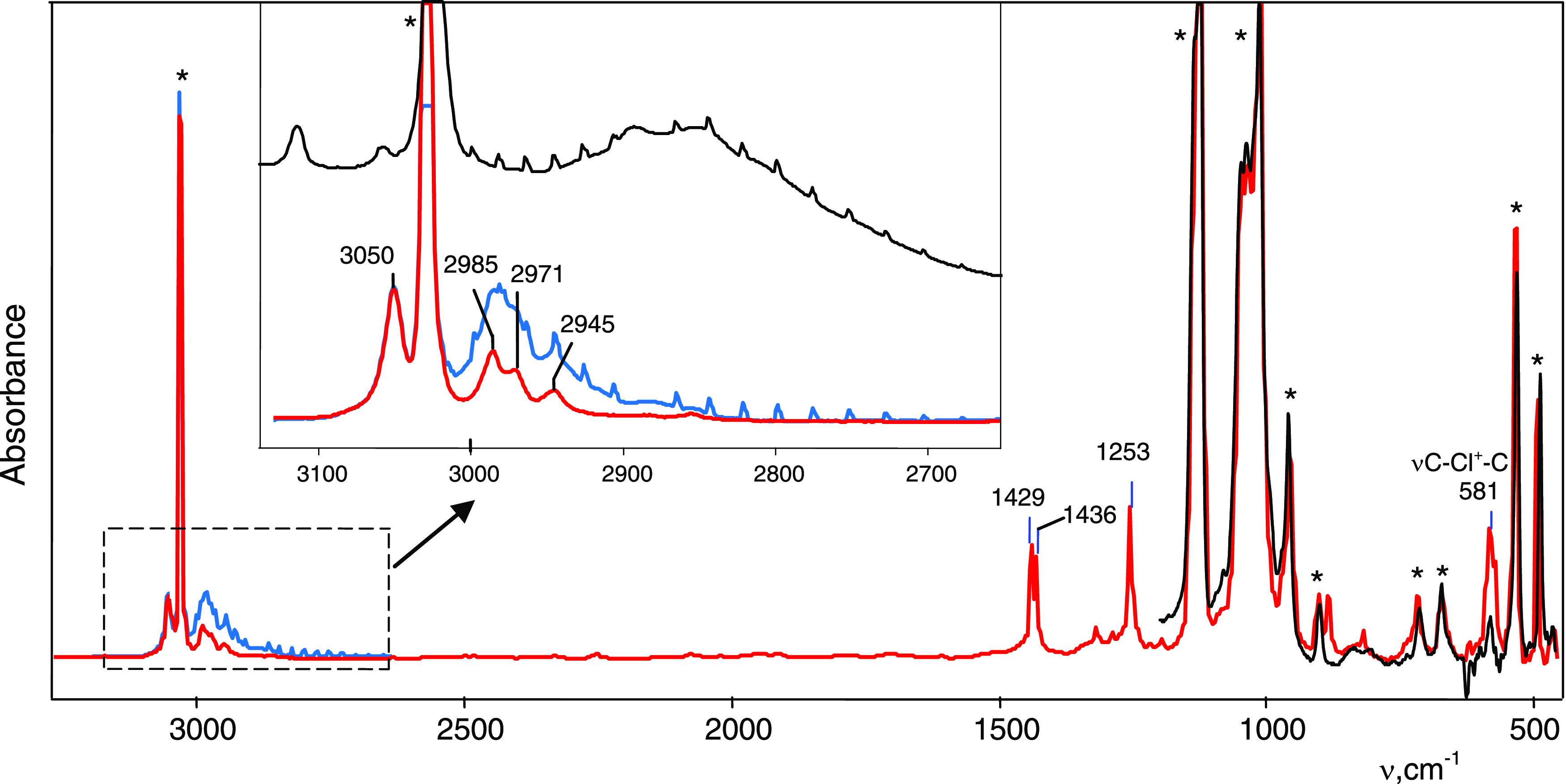
IR spectrum of products of the reaction of 1,4-dichlorobutane vapors with the H{Cl11} acid (blue) and an isolated spectrum of the formed salt (CH2CH2)2Cl+{Cl11–} (red), after the removal of 1,4-dichlorobutane vapors and gaseous HCl by evacuation. The spectrum of decomposition products of the salt (CH2CH2)2Cl+{Cl11–} when heated at 150 °C is black. The most distinctive bands of the {Cl11–} anion are marked with asterisks.
