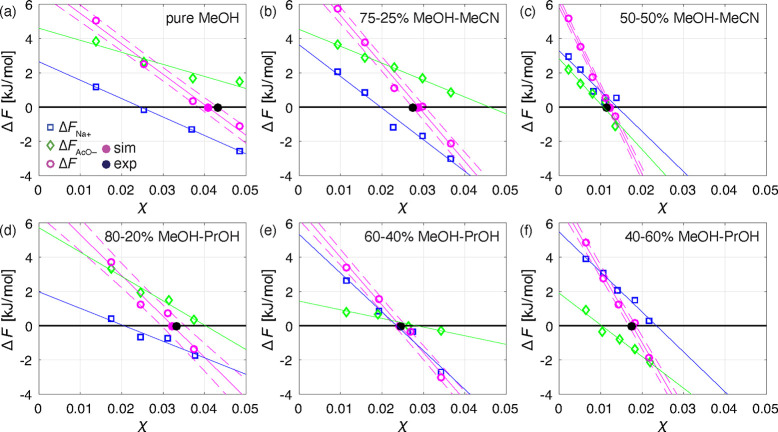
Figure 3.
Sampled energy differences between grown and solvated kink sites of Na+, ΔFNa+ (blue boxes), and AcO–, ΔFAcO– (green diamonds), as well as the dimeric unit, ΔF = ΔFNa+ + ΔFAcO– (purple circles), as a function of NaOAc mole fraction, χ, and compared to the experimental solubility values (black filled circles). Straight purple lines represent the linear regression of ΔF and the dashed lines are the corresponding standard deviations. The blue and green straight lines are the linear regressions of ΔFNa+ and ΔFAcO–, respectively. The predicted solubilities (purple filled circles) correspond to the mole fraction at ΔF = 0 obtained through a linear regression of ΔF as a function of χ. The results are shown for the systems of crystalline NaOAc exposed to following solutions: (a) pure MeOH; (b) 75–25% MeOH–MeCN; (c) 50–50% MeOH–MeCN; (d) 80–20% MeOH–PrOH; (e) 60–40% MeOH–PrOH; (f) 40–60% MeOH–PrOH. It is important to note that some of the presented ΔFNa+ and ΔFAcO– points are the averages of simulation repetitions (see SI Section S6).