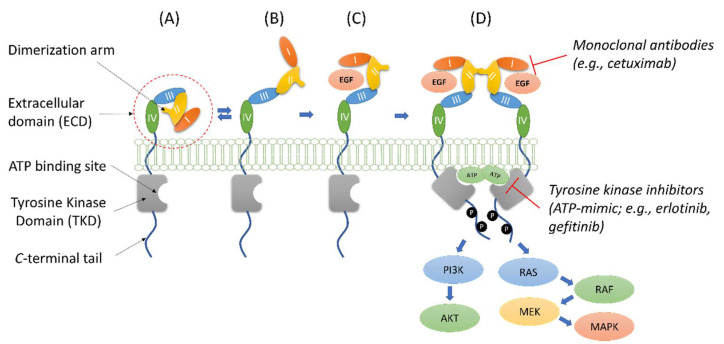
Figure 1.
Structure and simplified dynamics representation of EGFR. The auto-tethered fully inactive conformation of EGFR (A) is in equilibrium with a partially opened state (B) that can be stabilized by the binding with EGF (C). The EGF-activated conformation can dimerize (D) leading to receptor autophosphorylation and activation of the AKT and MAPK cascades that mediate cell survival and proliferation. The target regions for monoclonal antibodies and ATP-mimic compounds are also reported in (D).