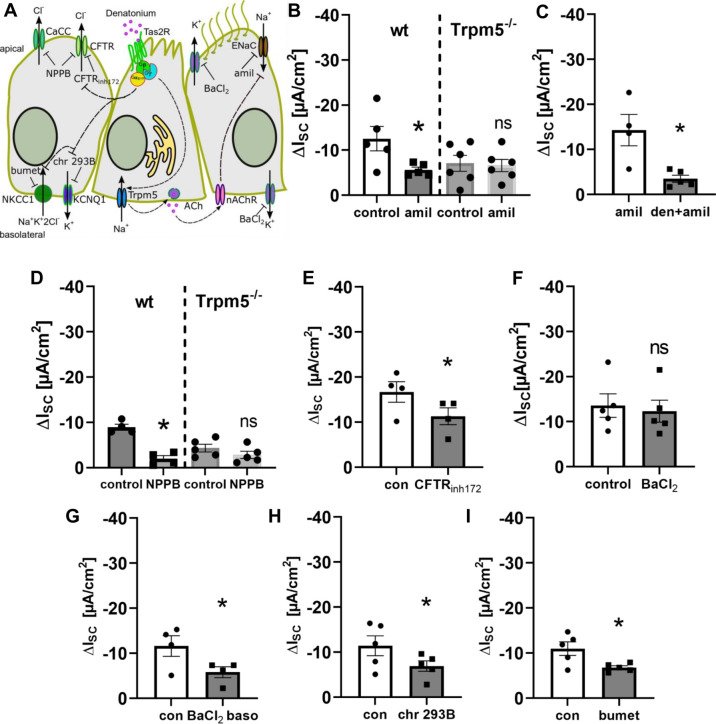
Figure 8.
Involvement of ion channels in the denatonium-induced effect. (A) Schematic drawing of the influence of the epithelial sodium channel (ENaC) inhibitor amiloride, the Cl−-channel antagonist NPPB, the cystic fibrosis transmembrane conductance regulator (CFTR) blocker CFTRinh172, and the K+-channel inhibitor BaCl2 and the KCNQ1 channel antagonist chromanol 293B as well as the inhibitor of the Na-K-2Cl cotransporter 1 (NKCC1) bumetanide. (B) The ENaC inhibitor amiloride (10 µM, apical) reduced the denatonium-induced current in wildtype but not in Trpm5−/− mice (ΔISC, n = 5–6, * p < 0.05, ns: not significant). (C) The effect of amiloride (10 µM, apical) was significantly reduced in the presence of denatonium (1 mM apical) (ΔISC, n = 4–5, * p < 0.05). (D) The Cl−-channel inhibitor NPPB (100 µM, apical) reduced the denatonium-induced effect in wildtype but not in Trpm5−/− mice (ΔISC, n = 5–6, * p < 0.05, ns: not significant). (E) The CFTR antagonist CFTRinh172 (10 µM, apical) reduced the denatonium-induced current (∆ISC, n = 4, * p < 0.05, con: control). (F) The non-selective K+-channel inhibitor BaCl2 (5 mM, apical) did not change the denatonium-induced current (∆ISC, n = 5, ns: not significant) compared to the control effect. (G) In the presence of 5 mM BaCl2 on the basolateral side of the epithelium, the denatonium-induced current was reduced (∆ISC, n = 4, * p < 0.05, con: control). (H) The KCNQ1 antagonist chromanol 293B (100 µM, basolateral, chr 293B) reduced the denatonium-induced current (∆ISC, n = 5, * p < 0.05, con: control). (I) The NKCC inhibitor bumetanide (200 µM, basolateral) reduced the denatonium-induced current (∆ISC, n = 5, * p < 0.05, con: control).