Figure 6.
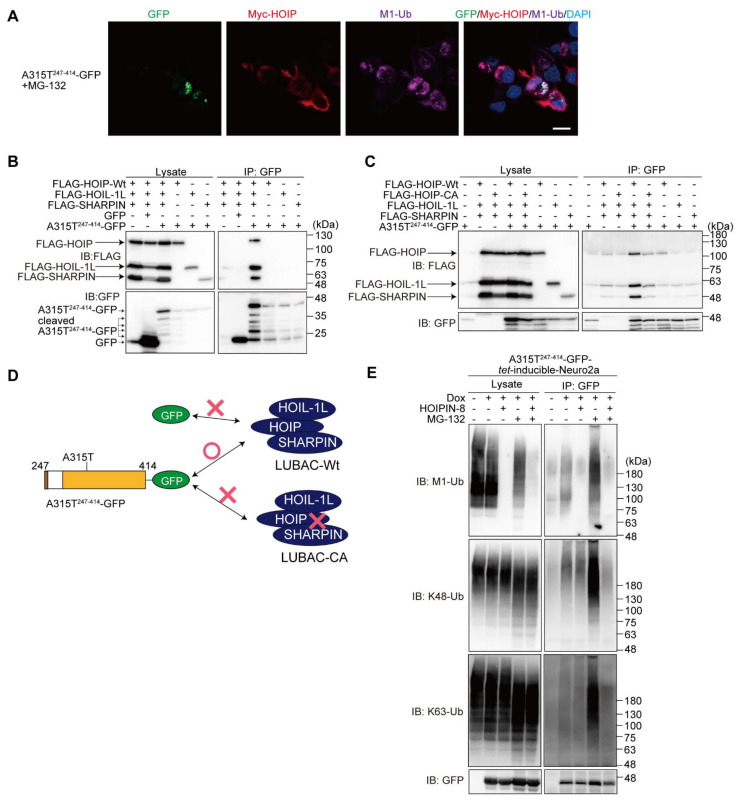
LUBAC activity is necessary for binding truncated TDP-43. (A) Colocalization of TDP-43 aggregates, LUBAC, and M1-ubiquitin. After the overexpression of LUBAC and A315T247-414-GFP in the presence of MG-132, immunofluorescence analyses of GFP, Myc-HOIP, and M1-ubiquitin were performed. Bar = 10 μm. (B) A315T247-414-GFP binds the LUBAC complex. FLAG-tagged LUBAC subunits, GFP, and/or A315T247-414-GFP were transfected into the HEK293T cells as indicated. Cell lysates and anti-GFP immunoprecipitates were immunoblotted with the indicated antibodies. (C) A315T247-414-GFP binds active LUBAC. A similar analysis as in (B) was performed, using WT or the active site C885A mutant of human HOIP, HOIP-CA. (D) A scheme of the truncated TDP-43-LUBAC binding. The results of (B,C) are summarized. (E) The effects of LUBAC and proteasome inhibitors on the M1-, K48-, and K63-ubiquitinations of A315T247-414-GFP. The A315T247-414-GFP-tet-inducible Neuro2a cells were treated with 1 μg/mL Dox and 10 μM HOIPIN-8 for 18 h, with 2 μM MG-132 added for the last 6 h, as indicated. Cell lysates obtained by the hot-SDS method and anti-GFP immunoprecipitates were immunoblotted with the indicated antibodies.