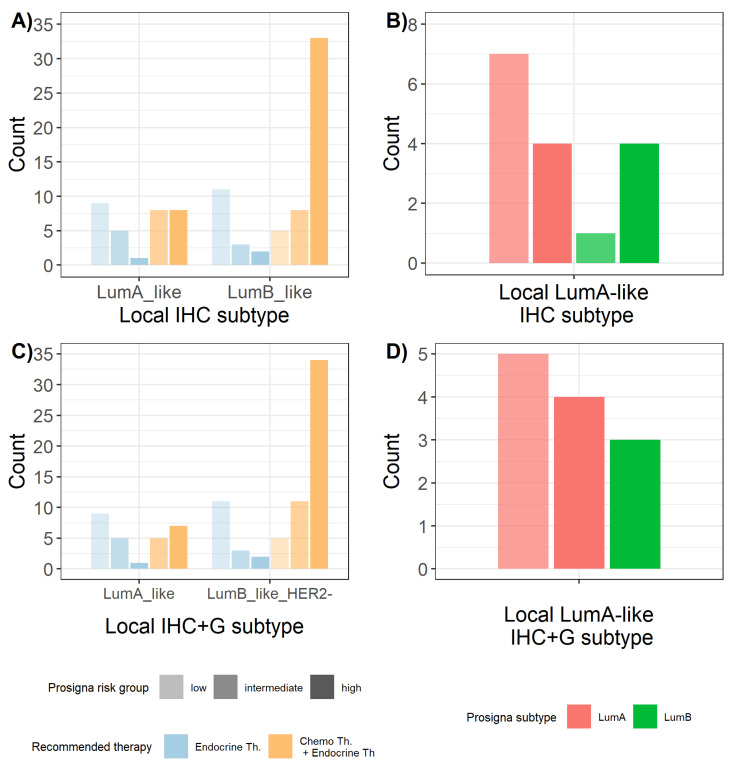
Figure 5.
Influence of Prosigna® results on treatment decision for locally assessed IHC and IHC+G surrogate subtypes. (A) Recommended therapy within locally assessed IHC surrogate subtypes and (B) distribution of Prosigna® subtype within the subset of Luminal A-like IHC subtypes that were recommended towards adjuvant chemotherapy and endocrine therapy. (C) Recommended therapy within locally assessed IHC+G surrogate subtypes and (D) distribution of Prosigna® subtype within the subset of Luminal A-like IHC+G subtypes that were recommended towards adjuvant chemotherapy and endocrine therapy. Colour intensity reflects Prosigna® risk group. IHC = immunohistochemistry; IHC+G = immunohistochemistry + tumor grade; LumA = Luminal A; LumB = Luminal B; Th. = therapy.