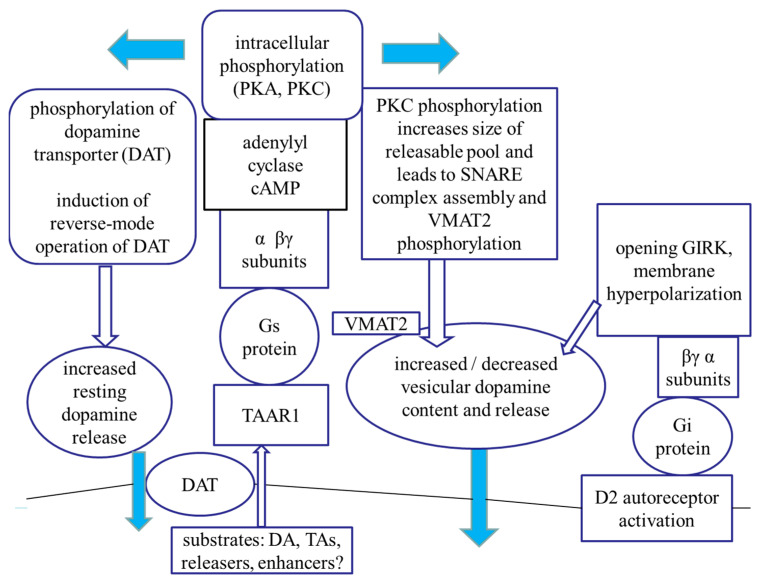
Figure 23.
Hypothetical model of the trace amine-associated receptor 1 (TAAR1) signaling involved in the regulation of presynaptic dopaminergic neurotransmission. Activation of the Gs protein-coupled TAAR1 by classical or trace amines and exogenous releaser drugs (amphetamines) results in adenylyl cyclase activation followed by an increased intracellular cAMP production and, as a consequence, an increase in PKA/PKC-mediated intracellular phosphorylation. Activation of PKC phosphorylates plasma membrane dopamine transporter (DAT) as well as proteins involved in SNARE core complex assembly leading to non-vesicular and vesicular dopamine release, respectively. Moreover, PKC also phosphorylates vesicular monoamine transporter 2 (VMAT2) and the increased dopamine accumulation causes elevated vesicular dopamine content and release. Dopamine released into the synaptic cleft activates D2 autoreceptor-mediated presynaptic feedback inhibition, which then leads to the opening of GIRK and the resulted hyperpolarization of the cell membrane suspends further release of dopamine. Our working hypothesis is that TAAR1 possesses a central role in the regulation of DAT, dopamine-containing vesicle operation, readily releasable pool and SNARE core complex assembly and feedback inhibition of dopamine release also. We have speculated that the enhancer drugs ((−)BPAP) activate TAAR1 signaling and the reserpine-sensitive PKC pool and phosphorylation of the proteins in SNARE core complex and VMAT2 leads to increased vesicular dopamine release, i.e., enhancer effect.