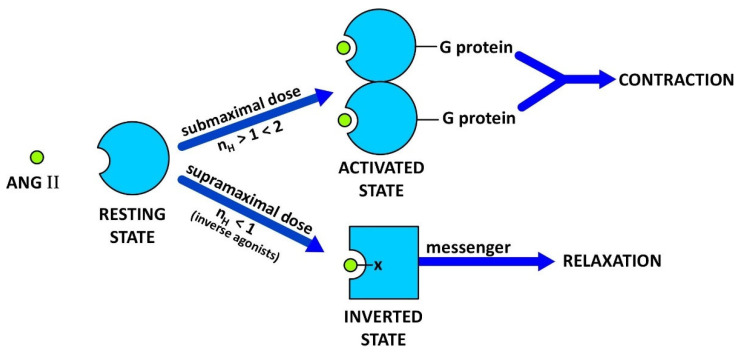
Figure 1.
Receptor switching from an agonist induced state to a desensitized inverse agonist state. Binding of submaximal doses of ANG II to its receptor induces G protein binding and dimerization of the receptor, a concerted mechanism of positive cooperativity (Hill coefficient nH > 1) leading to an increase in agonist affinity and consequent amplification of the contractile response. Different analogues may invoke different levels of cooperativity (kick up the affinity) more than others, e.g., weak or partial agonists. The maximum response may be limited by the available supply of G protein, without which the mode of receptor binding of ANG II [at supramaximal doses] changes to state#2 and induces negative cooperativity (nH < 1) synonymous with inverse agonism (tachyphylaxis). Thus, at high doses, ANG II itself becomes an inverse agonist [as do many partial agonists (2,4)]. Inverse agonists such as ARB drugs, [Sar1Ile8]ANG II, and angiotensin ‘antipeptides’ are unable to excite the receptor and bind to state#2 forming a salt bridge with Arg167 (shown as -X). The resulting insurmountable ‘inverted’ state of the angiotensin receptor engenders smooth muscle relaxation (vasodilation, via an alternative second messenger) for prolonged periods. This receptor-lockdown effect may be due to a slow rate of dissociation of the second messenger (see text).