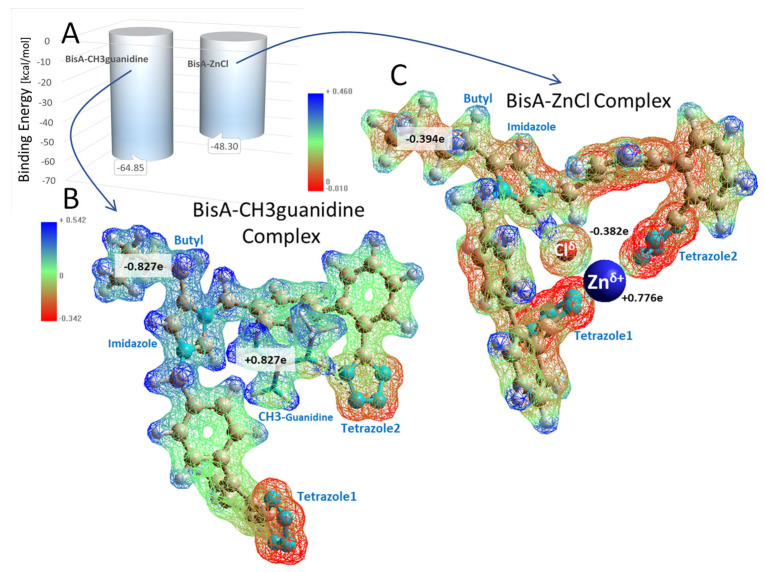
Figure 5.
Semiempirical RM1 calculation of intermolecular binding energies (A) for the BisA-CH3 guanidine and (B) BisA-ZnCl (C) complexes. Electrostatic potential distributions for each complex are shown below the graph. Note that lower (more negative) energies in this calculation indicate stronger binding. The RM1 calculations were carried out in in vacuo systems following energy minimization of the relaxed conformers shown in Figure 1, above. The total system charge for each complex was set to 0e for the Unrestricted Hartree–Fock (UHF) calculations. Geometry optimizations were performed by the Pollack–Ribiere conjugate gradient method with a final RMS gradient of 1.0 kcal/Å-mol and spin multiplicity of 1. The electrostatic potential maps (B,C) suggest the zinc ion (going from +2.0e to +0.776e) functioned as a stronger electron withdrawing group compared to CH3-guanidine (going from +1.0e to +0.827e). This effect was reflected in the total electron charge on the respective BisA molecules of each complex (see black-letter labels).