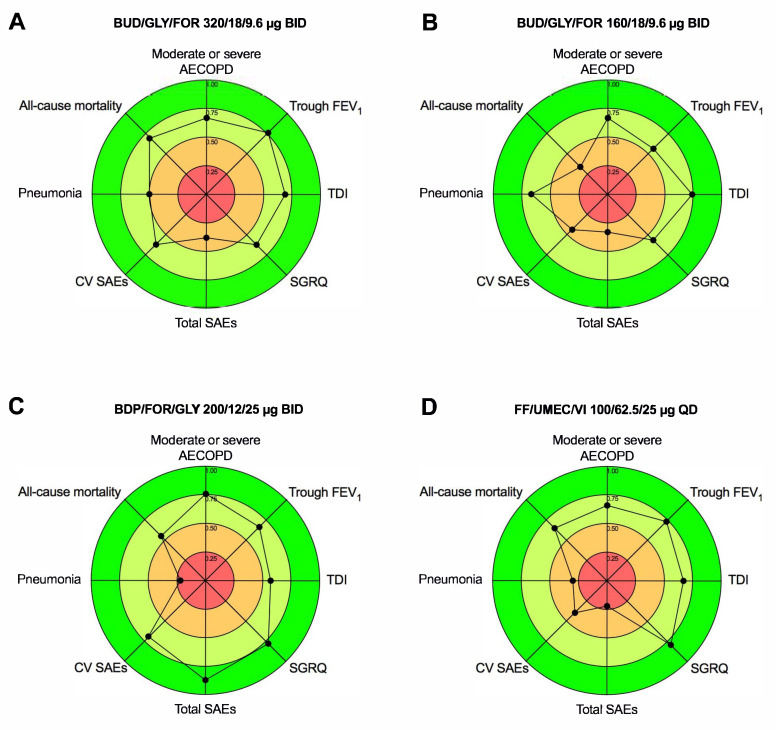
Figure 5.
Graphical representation of combined efficacy/safety profile of BUD/GLY/FOR 320/18/9.6 µg BID (A), BUD/GLY/FOR 160/18/9.6 µg BID (B), BDP/FOR/GLY 200/12/25 µg BID (C), and FF/UMEC/VI 100/62.5/25 µg QD (D) in COPD patients according to the IBiS score; a greater area indicates a better efficacy/safety profile. AECOPD: acute exacerbation of COPD; BDP: beclomethasone dipropionate; BID: bis in die, twice daily; BUD: budesonide; COPD chronic obstructive pulmonary disease; CV: cardiovascular; FDC: fixed-dose combination; FEV1: forced expiratory volume in the first second; FF: fluticasone furoate; FOR: formoterol fumarate; GLY: glycopyrronium bromide or glycopyrrolate; ICS: inhaled corticosteroid; IBiS: Implemented Bidimensional SUCRA; LABA: long-acting β2-adrenoceptor agonist; LAMA: long-acting muscarinic antagonist; QD: quaque die, once daily; SAEs: serious adverse events; SGRQ: St. George’s Respiratory Questionnaire; SUCRA: surface under the cumulative ranking curve; TDI: transitional dyspnea index; UMEC: umeclidinium bromide; VI: vilanterol.