Figure 4.
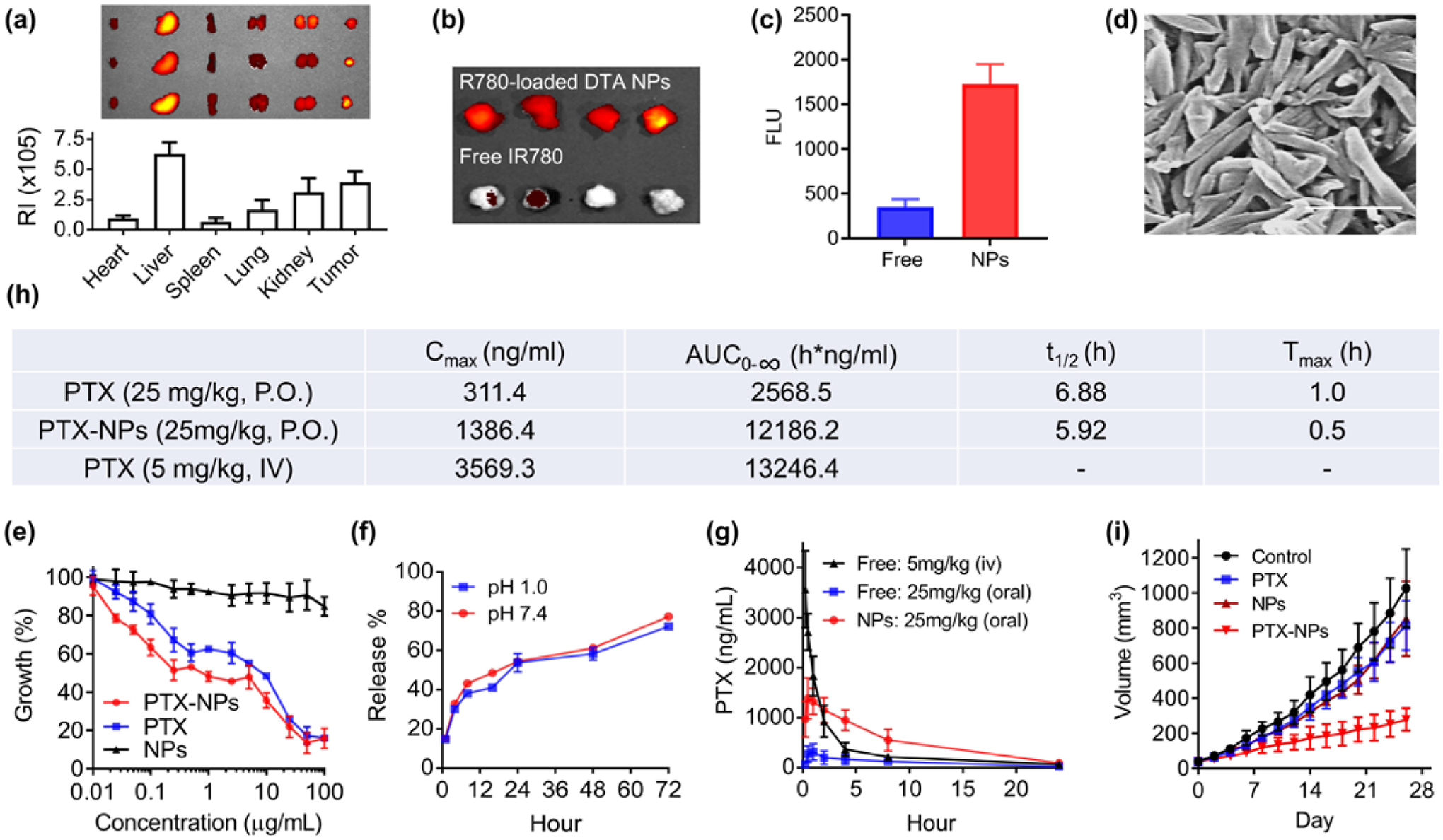
DTA NPs for oral delivery of PTX for cancer treatment. (a) Imaging and semi-quantification of DTA NPs in the indicated organs after oral administration. RI: Radiance efficiency (p sec−1cm−2sr−1uw−1cm−2), which was determined using Living Image 3.0 by dividing the fluorescence signal intensity with area of region of interest. (b) Representative fluorescence images of tumors isolated from mice receiving intravenous administration of IR-780 with and without encapsulation in DTA NPs. (c) Quantification of fluorescence intensities in mice received indicated treatments. (d) SEM characterization of PTX-loaded DTA NPs. Scale bar: 500 nm. (e) Toxicity of PTX, DTA NPs, and PTX-NPs on MDA-MB-231 cells. Cells were treated at the indicated concentrations. Growth of cells were determined 3 d after treatment by the standard MTT assay. (f) Release of PTX from NPs in PBS at the indicated pH 1.0 and pH 7.4 at 37°C. (g) Mean plasma concentration of PTX – time curves of PTX, DTA NPs, and PTX-loaded DTA NPs. (h) Pharmacokinetic parameters of PTX in the indicated formulations after oral administration. (i) Changes in tumor volume over time in mice receiving the indicated treatments (n = 6).
