Abstract
Background
Temporomandibular disorders (TMDs) are a group of musculoskeletal disorders affecting the jaw. They are frequently associated with pain that can be difficult to manage and may become persistent (chronic). Psychological therapies aim to support people with TMDs to manage their pain, leading to reduced pain, disability and distress.
Objectives
To assess the effects of psychological therapies in people (aged 12 years and over) with painful TMD lasting 3 months or longer.
Search methods
Cochrane Oral Health's Information Specialist searched six bibliographic databases up to 21 October 2021 and used additional search methods to identify published, unpublished and ongoing studies.
Selection criteria
We included randomised controlled trials (RCTs) of any psychological therapy (e.g. cognitive behaviour therapy (CBT), behaviour therapy (BT), acceptance and commitment therapy (ACT), mindfulness) for the management of painful TMD. We compared these against control or alternative treatment (e.g. oral appliance, medication, physiotherapy).
Data collection and analysis
We used standard methodological procedures expected by Cochrane. We reported outcome data immediately after treatment and at the longest available follow‐up.
We used the Cochrane RoB 1 tool to assess the risk of bias in included studies. Two review authors independently assessed each included study for any risk of bias in sequence generation, allocation concealment, blinding of outcome assessors, incomplete outcome data, selective reporting of outcomes, and other issues. We judged the certainty of the evidence for each key comparison and outcome as high, moderate, low or very low according to GRADE criteria.
Main results
We identified 22 RCTs (2001 participants), carried out between 1967 and 2021. We were able to include 12 of these studies in meta‐analyses. The risk of bias was high across studies, and we judged the certainty of the evidence to be low to very low overall; further research may change the findings. Our key outcomes of interest were: pain intensity, disability caused by pain, adverse events and psychological distress. Treatments varied in length, with the shortest being 4 weeks. The follow‐up time ranged from 3 months to 12 months. Most studies evaluated CBT.
At treatment completion, there was no evidence of a benefit of CBT on pain intensity when measured against alternative treatment (standardised mean difference (SMD) 0.03, confidence interval (CI) ‐0.21 to 0.28; P = 0.79; 5 studies, 509 participants) or control (SMD ‐0.09, CI ‐0.30 to 0.12; P = 0.41; 6 studies, 577 participants). At follow‐up, there was evidence of a small benefit of CBT for reducing pain intensity compared to alternative treatment (SMD ‐0.29, 95% CI ‐0.50 to ‐0.08; 5 studies, 475 participants) and control (SMD ‐0.30, CI ‐0.51 to ‐0.09; 6 studies, 639 participants).
At treatment completion, there was no evidence of a difference in disability outcomes (interference in activities caused by pain) between CBT and alternative treatment (SMD 0.15, CI ‐0.40 to 0.10; P = 0.25; 3 studies, 245 participants), or between CBT and control/usual care (SMD 0.02, CI ‐0.21 to 0.24; P = 0.88; 3 studies, 315 participants). Nor was there evidence of a difference at follow‐up (CBT versus alternative treatment: SMD ‐0.15, CI ‐0.42 to 0.12; 3 studies, 245 participants; CBT versus control: SMD 0.01 CI ‐ 0.61 to 0.64; 2 studies, 240 participants).
There were very few data on adverse events. From the data available, adverse effects associated with psychological treatment tended to be minor and to occur less often than in alternative treatment groups. There were, however, insufficient data available to draw firm conclusions.
CBT showed a small benefit in terms of reducing psychological distress at treatment completion compared to alternative treatment (SMD ‐0.32, 95% CI ‐0.50 to ‐0.15; 6 studies, 553 participants), which was maintained at follow‐up (SMD ‐0.32, 95% CI ‐0.51 to ‐0.13; 6 studies, 516 participants). For CBT versus control, only one study reported results for distress and did not find evidence of a difference between groups at treatment completion (mean difference (MD) 2.36, 95% CI ‐1.17 to 5.89; 101 participants) or follow‐up (MD ‐1.02, 95% CI ‐4.02 to 1.98; 101 participants).
We assessed the certainty of the evidence to be low or very low for all comparisons and outcomes.
The data were insufficient to draw any reliable conclusions about psychological therapies other than CBT.
Authors' conclusions
We found mixed evidence for the effects of psychological therapies on painful temporomandibular disorders (TMDs). There is low‐certainty evidence that CBT may reduce pain intensity more than alternative treatments or control when measured at longest follow‐up, but not at treatment completion. There is low‐certainty evidence that CBT may be better than alternative treatments, but not control, for reducing psychological distress at treatment completion and follow‐up. There is low‐certainty evidence that CBT may not be better than other treatments or control for pain disability outcomes.
There is insufficient evidence to draw conclusions about alternative psychological therapeutic approaches, and there are insufficient data to be clear about adverse effects that may be associated with psychological therapies for painful TMD.
Overall, we found insufficient evidence on which to base a reliable judgement about the efficacy of psychological therapies for painful TMD. Further research is needed to determine whether or not psychological therapies are effective, the most effective type of therapy and delivery method, and how it can best be targeted. In particular, high‐quality RCTs conducted in primary care and community settings are required, which evaluate a range of psychological approaches against alternative treatments or usual care, involve both adults and adolescents, and collect measures of pain intensity, pain disability and psychological distress until at least 12 months post‐treatment.
Plain language summary
What are the benefits and risks of psychological therapies for adults and young people over 12 years old with painful temporomandibular disorders (TMDs)?
Key messages
The overall results are mixed, but indicate that psychological therapies may be a useful approach for painful TMD as there is some limited evidence that they can reduce the pain. Our review suggests that they may do this at least as well as other available treatments. Any negative effects of psychological therapies are unclear, and more research is needed before we can know whether they provide a noticeable benefit while causing no or few problems.
What is the condition?
Temporomandibular disorders (TMDs) are conditions that affect the jaw joint and the muscles that move it. They are often associated with pain that lasts more than 3 months (known as chronic pain). Other symptoms include limited mouth opening, and jaw clicking and locking. All symptoms can interfere with quality of life and mood.
What did we want to know?
We wanted to find out how effective psychological therapies are for adults and young people over the age of 12 years who have painful TMD that has lasted at least 3 months.
What did we do?
We searched databases of medical and dental journals and research studies. We only selected studies known as 'randomised controlled trials (RCTs)'. In this type of study, participants are allocated to groups randomly. One group receives the intervention and the other receives a different treatment or no treatment at all. RCTs aim to reduce the risk of introducing bias in clinical studies.
We looked for reports of RCTs of psychological therapies compared to different treatments or no treatment in people over 12 years of age. Most of the reports we found compared psychological therapy to medication or the use of a special mouthguard.
We chose to focus on three measures of success. These were reduction in pain intensity, interference with activities caused by pain ('pain disability'), and psychological distress. We looked for details of these measures immediately after treatment and a few months later. We also looked for information on any 'adverse effects' (negative side effects of the treatments).
We used standard Cochrane methods to decide which studies to include, collect the key information from the studies, judge whether or not the studies were biased in any way, and judge how certain we can be about the results.
What did we find?
Overall we found 22 relevant studies. Most of the studies reported on one particular form of psychological therapy called cognitive behaviour therapy (CBT). We did not have enough information to draw any conclusions about any other psychological therapies.
The results told us that CBT was no different to other treatments (e.g. oral splints, medicine) or usual care/no treatment in reducing the intensity of the TMD pain by the end of treatment. There was some evidence that people who had CBT might have slightly less pain a few months after treatment.
There was some evidence that CBT might be better than other treatments for reducing psychological distress both at the end of treatment and a few months later. This was not seen in the one study that compared CBT against usual care.
In terms of how much pain interfered with activities, there was no evidence that there was any difference between CBT and other treatments.
There was too little information to be sure about whether psychological treatments cause adverse effects (problems caused by treatment such as feeling unwell or worse pain or unexpected effects). Only six of the 22 studies measured what adverse effects participants experienced. In these six studies, adverse effects associated with psychological treatment seemed to be minor in general and to occur less often than in alternative treatment groups.
What are the limitations of the evidence?
We have little confidence in the evidence because many of the studies had design limitations. There was also variation in the length of treatment and in how it was delivered. This means that we need to be cautious in interpreting the results that we found and they may not be reliable.
How up to date is the evidence?
We searched for studies up to 21 October 2021.
Summary of findings
Summary of findings 1. CBT versus alternative active intervention for painful TMD.
| CBT compared with alternative treatment for painful TMD | ||||
|
Population: people with painful TMD Settings: any primary, secondary or tertiary care setting Intervention: CBT Comparison: alternative treatment | ||||
| Outcomes | Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments |
|
Pain intensity at treatment completion Pain intensity measured by multiple scales including CPI, BPI, McGill Pain Questionnaire, VAS or numerical rating scales. Higher scores indicate higher pain intensity. |
Mean pain intensity in the CBT group was 0.03 SDs higher (0.21 lower to 0.28 higher) | 509 (5 RCTs) | ⊕⊕⊝⊝ Lowa | |
|
Pain intensity at follow‐up (6‐12 months) Pain intensity measured by multiple scales including CPI, BPI, McGill Pain Questionnaire, MPI, VAS or numerical rating scales. Higher scores indicate higher pain intensity. |
Mean pain intensity in the CBT group was 0.29 SDs lower (0.50 lower to 0.08 lower) | 475 (5 RCTs) | ⊕⊕⊝⊝
Lowa |
|
|
Disability caused by pain at treatment completion Measured by multiple scales including GCPS, RMDQ, PSEQ, OHIP, PDI, numerical rating scales. Higher scores indicate higher disability. |
Mean disability in the CBT group was 0.15 SDs lower (0.40 lower to 0.10 higher) | 245 (3 RCTs) | ⊕⊕⊝⊝
Lowa |
|
|
Disability caused by pain at follow‐up Measured by multiple scales including GCPS, RMDQ, PSEQ, OHIP, PDI, numerical rating scales. Higher scores indicate higher disability. |
Mean disability in the CBT group was 0.15 SDs lower (0.42 lower to 0.12 higher) | 245 (3 RCTs) | ⊕⊕⊝⊝
Lowa |
|
| Adverse events | 3 studies reported minor adverse events in the control group; 1 study reported minor adverse events in psychological therapy group; 1 study reported that there were no adverse events | (5 RCTs) | ⊕⊝⊝⊝ Very lowb | |
|
Psychological distress at treatment completion Measured by multiple scales including PHQ‐9, CES‐D, BDI, SF‐36, SCL‐90 Higher scores indicate higher distress. |
Mean psychological distress in the CBT group was 0.32 SDs lower (0.50 lower to 0.15 lower) | 553 (6 RCTs) | ⊕⊕⊝⊝
Lowa |
|
|
Psychological distress at follow‐up Measured by multiple scales including PHQ‐9, CES‐D, BDI, SF‐36, SCL‐90 Higher scores indicate higher distress. |
Mean psychological distress in the CBT group was 0.32 SDs lower (0.51 lower to 0.13 lower) | 516 (6 RCTs) | ⊕⊕⊝⊝
Lowa |
|
| BDI: Beck Depression Inventory; BPI: Brief Pain Inventory; CBT: cognitive behaviour therapy; CES‐D: Centre for Epidemiological Studies in Depression; CPI: Characteristic Pain Intensity; GCPS: Graded Chronic Pain Scale; OHIP: Oral Health Impact Scale; MPI: Multidimensional Pain Inventory; PDI: Pain Disability Index; PHQ‐9: Patient Health Questionnaire; PSEQ: Pain Self‐Efficacy Questionnaire; RCT: randomised controlled trial; RMDQ: Roland‐Morris Disability Questionnaire; RMPQ: Roland‐Morris Disability Questionnaire; SD: standard deviation; SF‐36: Short Form‐36; SCL‐90: Symptom Checklist 90 Revised; TMD: temporomandibular disorder; VAS: visual analogue scale | ||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||
aDowngraded by two levels for risk of bias and imprecision. bDowngraded by three levels for inconsistency, risk of bias and imprecision.
Summary of findings 2. CBT versus control for painful TMD.
| CBT compared with usual care or no‐treatment control for painful TMD | ||||
|
Population: people with painful TMD Settings: any primary, secondary or tertiary care setting Intervention: CBT Comparison: usual care, waiting list or no treatment | ||||
| Outcomes | Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments |
|
Pain intensity at treatment completion Measured by multiple scales including CPI, BPI, McGill Pain Questionnaire, VAS or numerical rating scales Higher scores indicate higher pain intensity. |
Mean pain intensity in the CBT group was 0.09 SDs lower (0.30 to 0.12 lower) | 577 (6 RCTs) | ⊕⊕⊝⊝
Lowa |
|
|
Pain intensity at follow‐up (12 months) Measured by multiple scales including CPI, BPI, McGill Pain Questionnaire, VAS or numerical rating scales Higher scores indicate higher pain intensity. |
Mean pain intensity in the CBT group was 0.30 SDs lower (0.51 to 0.09 lower) | 639 (6 RCTs) | ⊕⊕⊝⊝
Lowa |
|
|
Pain disability at treatment completion Pain disability or interference measured by multiple scales including GCPS, RMDQ, PSEQ, OHIP, PDI, numerical rating scales. Higher scores indicate higher disability. |
Mean disability in the CBT group was 0.02 SDs higher (0.21 lower to 0.24 higher) | 315 (3 RCTs) | ⊕⊕⊝⊝
Lowa |
|
|
Pain disability at follow‐up Pain disability or pain interference measured by multiple scales including GCPS, RMDQ, PSEQ, OHIP, PDI, numerical rating scales. Higher scores indicate higher disability. |
Mean disability in the CBT group was 0.01 SDs higher (0.61 lower to 0.64 higher) | 240 (2 RCTs) | ⊕⊕⊝⊝
Lowa |
|
| Adverse events | Only 1 study reported adverse events as an outcome: it found worsening symptoms in 13 people treated with an oral splint (as part of standard treatment) and 4 people treated with CBT. | 101 (1 RCT) | ⊕⊝⊝⊝ Very lowb | |
|
Psychological distress at treatment completion Measured by multiple scales including PHQ‐9, CES‐D, BDI, SF‐36, SCL‐90. Higher scores indicate higher distress. |
MD 2.36, 95% CI ‐1.17 to 3.89 | 101 (1 RCT) | ⊕⊕⊝⊝
Lowa |
|
|
Psychological distress at follow‐up Measured by multiple scales including PHQ‐9, CES‐D, BDI, SF‐36, SCL‐90 Higher scores indicate higher distress. |
MD ‐1.02, 95% CI ‐4.02 to 1.98 | 101 (1 RCT) | ⊕⊕⊝⊝ Lowa |
|
| BDI: Beck Depression Inventory; BPI: Brief Pain Inventory; CBT: cognitive behaviour therapy; CES‐D: Centre for Epidemiological Studies in Depression; CPI: Characteristic Pain Intensity; GCPS: Graded Chronic Pain Scale; OHIP: Oral Health Impact Scale; PDI: Pain Disability Index; PHQ‐9: Patient Health Questionnaire; PSEQ: Pain Self‐Efficacy Questionnaire; RCT: randomised controlled trial; RMDQ: Roland‐Morris Disability Questionnaire; SF‐36: Short Form‐36; SCL‐90: Symptom Checklist 90 Revised; SD: standard deviation; SMD: standardised mean difference; TMD: temporomandibular disorder; VAS: visual analogue scale | ||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||
aDowngraded by two levels for risk of bias and imprecision or inconsistency. bDowngraded by three levels for inconsistency, risk of bias and imprecision.
Background
Description of the condition
Temporomandibular disorders (TMDs) are a group of musculoskeletal conditions affecting the muscles of mastication, temporomandibular joints, and associated tissues (Durham 2015; Greene 2010). They are frequently painful and are the second‐most common cause of pain after low back pain (NIDCR 2019), affecting 5% to 12% of people internationally (Sharma 2018). The term, TMD, covers a range of diagnostic subtypes, including the most common: muscle‐related pain (myalgia, local myalgia, myofascial pain, myofascial pain with referral), joint or disc‐related problems (arthralgia and varying types of disc displacements), and headache attributed to TMD (Schiffman 2014). Clinical signs and symptoms of TMD occur in and around the jaw area, which may also spread throughout the face. Painful TMD may include pain originating from the muscles, articular disc, or jaw joint, and may involve a range of signs and symptoms, including joint sounds, headache, local or diffuse pain, and restricted mobility of the temporomandibular joint (Beecroft 2019).
The first onset incidence of TMD in adults is estimated at between 4% and 19% per annum (Slade 2013), and TMDs are estimated to be 1.5 to 2 times more common in women compared to men (Sharma 2018). Nearly half of people who experience pain continue to do so beyond 6 months (Slade 2016). TMD may be diagnosed based on an examination and history of clinical symptoms or may follow more operationalised diagnostic systems that have been developed, such as the Diagnostic Criteria for TMD (DC/TMD) (Schiffman 2014) or Research Diagnostic Criteria for TMD (RDC/TMD) (Dworkin 1992). TMD are commonly managed by a wide range of clinicians, including general dental and medical practitioners, and medical and dental specialists in secondary care.
TMDs are associated with symptoms including pain, limited mouth opening, and jaw clicking and locking; they are known to have significant impacts on quality of life and daily activities (Durham 2010), including particular challenges for communication, mastication, and intimacy (Durham 2007; Durham 2010). They are biopsychosocial in nature (Dworkin 1994; Ohrbach 2018), i.e. influenced by continual interaction of biological, psychological, and social elements. TMDs share characteristics with other persistent pain conditions, including distress and interference in everyday tasks and meaningful activities. Indeed, they are often comorbid with other pain conditions (Schiffman 2014). From a psychological perspective, pain, limitations to functional activities and impact on mood are of primary concern, and the psychological interventions applied are unlikely to differ substantially according to subtype of TMD.
Recommended treatment for people with TMD aged 12 or above in the UK is summarised by a National Institute for Health and Care Excellence (NICE) Clinical Knowledge Summary (NICE 2016). The guidelines recommend giving information and reassurance about the normal non‐progressive nature of the condition. Recommended advice to people at an early stage of TMD is to: restrict eating to a soft‐food diet; identify and address signs of stress; avoid parafunctional activities such as bracing, clenching, and wide yawning; relax the jaw; and manage the pain with heat, ice, and over‐the‐counter medications. The guidelines also suggest that for people with TMD who have a habit of clenching or grinding their teeth, an oral appliance (also referred to as an oral splint) may be appropriate. Dentists frequently use oral splints as a first‐line treatment (Aggarwal 2012). Once pain has lasted beyond 3 months, it is defined as chronic (or persistent) (Treede 2015). At this stage, pain is likely to be maintained by an interaction of biopsychosocial factors regardless of the original cause of the pain (Loeser 1999); psychological treatments are therefore likely to be helpful at this stage.
The NICE 2016 Clinical Knowledge Summary recommends referral for psychological input if the person with TMD has co‐existing anxiety or high levels of distress (NICE 2016). However, psychological treatment for management of pain does not only focus on reducing anxiety and depression. It can also be part of a broader management strategy that has alleviation of pain and disability as key treatment outcomes (Williams 2012). Psychological treatment will often target improved self‐management as a means to achieve reductions in pain and disability. Typically, interventions are likely to be individual or group‐based behavioural or cognitive‐behavioural therapies delivered over a fixed period of between 4 and 12 sessions, which research studies evaluate against attention control or usual care. Interventions may include a wide variety and number of components, all of which have been designed to reduce pain, reduce the disability and distress associated with pain, or to increase adaptive behaviours in the presence of pain, using psychological principles.
Description of the intervention
Psychological therapies refer to a broad range of interventions, which can be delivered individually or in group settings. They are informed by theories of human behaviour (Williams 2012), and usually involve a combination of education and development of new cognitive and behavioural skills, which are introduced to patients and then practised in real‐life settings. Psychological therapies are often delivered as part of a multidisciplinary approach alongside other non‐pharmacological treatments including physiotherapy (Paço 2016), oral splints (Singh 2017), routine self‐management support (Palmer 2022; Story 2016), and also alongside pharmacological treatments, where these are indicated (Mujakperuo 2010). Evidence for the effectiveness of psychological therapies is weak or equivocal at present, with too few studies to draw definitive conclusions.
Psychological therapies for TMD commonly involve behavioural interventions (Fordyce 1968), alone or in conjunction with cognitive interventions (Turk 1983); a purely cognitive approach is rarely used. Cognitive behaviour therapy (CBT) is used most often (Turk 1983), and has some evidence of effectiveness (Randhawa 2016). Cognitive approaches aim to help people with TMD to evaluate their thoughts for accuracy and helpfulness, and to provide strategies for recognising and changing, or responding differently to, patterns of thinking or core beliefs that may be contributing to pain or distress. Behavioural approaches to pain management focus on changing what the person does; for example, teaching biofeedback or relaxation techniques, or using positive reinforcement to reduce behaviours that might exacerbate pain. Biofeedback involves relaxation alongside physiological feedback through an electronic device so that people are able to monitor how relaxed they are physiologically. We do not consider biofeedback, as a stand‐alone intervention, to represent a psychological therapy. We will, however, include studies that have biofeedback as a behavioural component alongside other psychological interventions.
Numerous psychological therapies that could potentially be helpful in TMD have been applied within more general persistent pain settings (Barker 2019), for example, ‘third‐wave' cognitive therapies, which focus on the context rather than the content of thoughts. Acceptance and commitment therapy (ACT) is one such 'third‐wave' therapy, which aims to change the way that people relate to their thoughts, sensations, and other internal experiences rather than trying to influence the experiences themselves (Dahl 2004; Hayes 1999); people are encouraged to pursue life goals that are important and meaningful to them, even if doing so involves experiencing difficult and painful feelings. Compassion‐focused therapy also aims to help people to relate differently to their internal experiences, using insights from evolutionary psychology to explain to people why they might have thoughts and engage in behaviours that could be unhelpful and difficult to understand, and then helping them to adopt a kinder, gentler, and therefore more helpful attitude towards their difficulties. This process then helps to interrupt habitual patterns of resistance that might otherwise intensify pain (Gooding 2020; Penlington 2019a). Cognitive functional therapy integrates methods associated with psychology and physiotherapy to develop a personalised understanding of factors that may be contributing to pain, leading to an evolving and personalised plan to reduce and change these factors (O'Sullivan 2012). Mindfulness is a method of staying with, or returning to, present‐moment experience that can also lead to changes in the habitual ways that people respond to difficulty such as pain or distress. Mindfulness will be included within this Cochrane Review as it is based on a psychology of human minds and how they work, and has been widely applied in pain management settings (Kabat‐Zinn 1982).
Frequently, psychological therapies are delivered as part of a broader biopsychosocial intervention by a multidisciplinary team of psychologists, physiotherapists, doctors, dentists, and other health professionals with specific expertise in the management of pain. The psychological components are delivered by specialist psychologists or other staff who have been trained and are supervised by psychologists. Psychological therapies for adults may also involve information or specific sessions targeted to family, significant others or carers of the individual with TMD. For adolescents under the age of 18, a systemic approach involving family would usually be an important component of psychological treatment.
Psychological therapies have a range of levels of intensity and formats for delivery. Increasingly these are delivered within a stepped care model which might, for example, include pure self‐help (step 1), guided self‐help or group therapy often delivered by a psychological well‐being practitioner or nurse (step 2), brief individual therapy (step 3), or more intensive individual therapy of longer duration (step 4) (Bower 2005). There is some early evidence that this approach can usefully be applied to psychological therapies for pain management (Bell 2020). There is some evidence in the field of TMD for matching psychological interventions to pain characteristics that might suggest the utility of a stepped care model. Psychological interventions may be more successful and require less intensive input for people who report pain that is less disabling (Dworkin 2002a), and of shorter duration (Gatchel 2006; Gatchel 2014), compared with longer‐standing and more disabling pain. The differential need for treatment according to individual factors, including disability, is established in general pain management services by initiatives such as the STarT Back trial (Hill 2011).
How the intervention might work
An important aim of psychological therapies for TMD is to support self‐management. Self‐management is recognised in national and international guidelines as the first‐line treatment for TMD (De Leeuw 2008; Durham 2015; Greene 2010). Self‐management refers to a person's use of a range of strategies to enable them to live well with pain, minimising pain where possible while also minimising its impact on life. Although it is considered to be an important aspect of living with pain, successful self‐management can be difficult to achieve. An instinctive response to pain is to try to fight or avoid it. When pain persists in the absence of a treatable cause or despite optimal medical management, these automatic responses can cause distress and may maintain and even increase the intensity of pain. Psychological therapies support self‐management by encouraging behaviours that are helpful and reducing responses that are potentially harmful, including helping to overcome barriers to effective engagement in self‐management, where necessary (Williams 2012). Targets of such therapies will depend on the theory on which they are based, but may include reducing anxiety or depression, modifying stress reactivity or reducing habitual behaviours, introducing effective coping strategies, increasing confidence and ability to engage in rewarding and meaningful activities, reframing the meaning of pain, or redirecting focus away from pain and towards valued life goals.
Why it is important to do this review
TMDs are common (painful) problems that have a significant impact on individuals and society (Sharma 2018). Clinically, a wide range of interventions are used to treat TMDs and there is a need for accurate scientific evidence to direct the use of such interventions. TMD can present with a wide range of severity and complexity, and different treatment approaches may be appropriate for different presentations. Clinical guidelines highlight the importance of self‐management from an early stage and alongside other treatment (Greene 2010). Support for self‐management is an approach that is psychological in nature, although this is not made explicit in the current literature relating to TMD. The self‐management support that is currently provided as part of routine care therefore lacks an evidence base, is highly variable, and is often delivered without psychological training or support.
Dentists, who are frequently the first point of contact for people with painful TMD, are often not confident about this aspect of management, partly due to a reported/perceived lack of training in TMD and persistent pain (Durham 2007). They also face organisational and training barriers to the delivery of appropriate education and support for self‐management (Peters 2015), and tend to prescribe an oral appliance and/or refer to dental specialists as a first line of treatment (Aggarwal 2012). Few providers of psychological therapies are integrated with, or have expertise in, orofacial pain, and where referrals are made to community psychology services or pain clinics, waiting times are often long. Potentially, therefore, current treatment provision may be suboptimal for people with TMD. There is some evidence that early psychological therapy targeted towards people with higher reported disability and distress associated with TMD can improve long‐term outcomes and reduce the chances of the pain persisting beyond a year (Gatchel 2014). More literature is available for psychological interventions for persistent pain in TMD at a higher level of intensity, usually for people with longer durations of pain, where weak evidence for psychological treatments is reported alongside the need for more high‐quality trials. The most recent review of this literature included primary literature published up to 2014 (Randhawa 2016). There is, therefore, a need for an updated comprehensive systematic review of psychological therapies for painful TMD to assist providers and commissioners in the planning and development of services to best meet the needs of people with TMD according to current scientific evidence.
Clinical practice guidelines for TMD start at age 12 years (NICE 2016). Although incidence of TMD is lower for adolescents than adults, presentations during adolescence do occur. Incidence of new‐onset TMD is known to increase between the ages of 12 and 19 years. A 3‐year longitudinal study in Sweden found that 11.4% of adolescents reported TMD pain on at least one occasion, though the proportion with chronic pain was less than 1% (Nilsson 2007). There is currently no available review of psychological therapies for TMD in adolescents, therefore there is a need to also consider evidence that may be available for psychological therapies in this population.
Objectives
To assess the effects of psychological therapies in people (aged 12 years and over) with painful TMD lasting 3 months or longer.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) of any psychological therapy (e.g. cognitive behaviour therapy (CBT), behaviour therapy (BT), acceptance and commitment therapy (ACT), mindfulness) for the management of painful TMD. We included cross‐over trials if the effects of each arm could be evaluated independently. Studies needed to be based on recognisable psychological theory, as judged by the review authors, and delivered by qualified psychologists or staff trained and supervised in the treatment approach specified.
Types of participants
Eligible participants were adults and adolescents aged 12 years or older, with pain lasting 3 months or longer since initial onset of TMD diagnosed by any method. We noted exclusion criteria and recorded physical and psychological comorbidities and use of medications. We recorded whether diagnosis was made by formalised criteria including DC/TMD, RDC/TMD, or other formal method, or whether this was not stated. We included studies of interventions directed towards a mixture of pain conditions so long as information about TMD was reported separately, or if the entire sample was reported to consist of 80% or more participants with TMD.
Types of interventions
Interventions
We included any psychological intervention for the management of TMD against any control condition. We defined psychological treatments as any interventions based on any widely accepted psychological theory and delivered by psychologists or staff with appropriate behavioural healthcare training. We included mixed intervention studies if we considered at least 50% of the treatment content to be psychological in nature. We excluded studies of treatment packages that include interventions, such as oral splints or medication, where it was not possible to independently evaluate the effects of the psychological component of the intervention.
We included interventions of any format and length that met our inclusion criteria.
Comparisons
We considered psychological interventions, i.e. CBT, BT or other psychological therapy, that were compared to an alternative treatment (such as medication or oral splints) or a control condition, such as usual care (as defined by trial authors), attention control (such as a support group that contained no active treatment but offered a similar amount of face‐to‐face contact as the active intervention), waiting‐list control, or no treatment. We also considered studies that evaluated one psychological intervention against another.
Types of outcome measures
Primary outcomes
Pain intensity, e.g. Characteristic Pain Intensity (CPI) (Von Korff 1992), single visual analogue scale (VAS) or numerical rating scales of pain (NRS), or quantitative measures based on pain descriptors such as the McGill Pain Questionnaire (MPQ) (Melzack 1975)
Disability caused by pain (pain impact), e.g. Graded Chronic Pain Scale (GCPS) (Von Korff 1992), Roland‐Morris Disability Questionnaire (RMDQ) (Roland 2000), Oral Health Impact Scale (Slade 1994) or Pain Self‐Efficacy Questionnaire (PSEQ) (Nicholas 2007)
Adverse events
Secondary outcomes
Psychological distress, e.g. Patient Health Questionnaire PHQ‐4 (Kroenke 2009), PHQ‐9 (Kroenke 2001), Hospital Anxiety and Depression (HAD) scale (Zigmond 1983), General Health Questionnaire GHQ‐12 (Goldberg 1977), Beck Depression Inventory‐II (BDI‐II) (Beck 1996), Beck Anxiety Inventory (BAI) (Beck 1988), or Perceived Stress Scale (PSS) (Cohen 1983)
Additional physical symptoms, e.g. Symptom Checklist SCL‐90‐R (Derogatis 1983), Patient Health Questionnaire PHQ‐15 (Kroenke 2002)
Quality of life, e.g. EQ‐5D‐5L (Oppe 2014)
These measures are self‐reported patient questionnaires. We planned to report outcomes regardless of whether these were self‐reported or observer‐rated; in practice, all included studies used self‐reported primary outcome measures. Where a study included more than one measure for a domain, we selected the measure that we judged most appropriate for that outcome, considering frequency of use of the measure in the field and reported reliability of the measure. Where follow‐up data from a study were provided at more than one point in time, we reported data for the longest follow‐up time available.
Search methods for identification of studies
Electronic searches
Cochrane Oral Health’s Information Specialist conducted systematic searches in the following databases for RCTs and controlled clinical trials. There were no language, publication year or publication status restrictions.
Cochrane Oral Health's Trials Register (searched 21 October 2021) (Appendix 1)
Cochrane Central Register of Controlled Trials (CENTRAL) in the Cochrane Register of Studies (searched 21 October 2021) (Appendix 2)
MEDLINE Ovid (1946 to 21 October 2021) (Appendix 3)
Embase Ovid (1980 to 21 October 2021) (Appendix 4)
PsycINFO Ovid (1806 to 21 October 2021) (Appendix 5)
Trip database (www.tripdatabase.com/) (searched 21 October 2021) (Appendix 6)
The subject strategies for databases were modelled on the search strategy designed for MEDLINE Ovid in Appendix 3. Where appropriate, this was combined with subject strategy adaptations of the highly sensitive search strategy designed by Cochrane for identifying RCTs and controlled clinical trials (as described in the Cochrane Handbook for Systematic Reviews of Interventions Box 6.4.c (Lefebvre 2020)).
Searching other resources
Cochrane Oral Health's Information Specialist searched for grey literature in the following databases.
Web of Science Conference Proceedings (1990 to 21 October 2021) (Appendix 7)
Proquest Dissertations and Theses Global (1861 to 21 October 2021) (Appendix 8)
OpenGrey (www.opengrey.eu/) (searched 21 October 2021) (Appendix 9)
The following trials registries were also searched.
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (clinicaltrials.gov/) (see Appendix 10)
World Health Organization International Clinical Trials Registry Platform (apps.who.int/trialsearch) (see Appendix 11)
We searched reference lists of included studies and review articles of psychological therapies for orofacial pain in general, and TMD specifically. We checked to ensure that none of the included studies have been retracted due to error or fraud. We did not perform a separate search for adverse effects of interventions. We considered adverse events described in included studies only.
Data collection and analysis
Selection of studies
Our strategy was to include RCTs, including cross‐over trials where the effects of each arm could be evaluated independently. Studies were of any psychological intervention for TMD. We designed the search to be sensitive and include controlled clinical trials, which we then filtered out early in the selection process if not randomised.
Five review authors (CP, CB, GT, AAO, PW) screened titles and abstracts of retrieved studies, and retained those that fitted the inclusion criteria or were not obviously excluded by the criteria. We obtained full‐text copies and at least two of three review authors (CP, CB, GT) independently judged whether each study fulfilled the inclusion criteria. We resolved differences in opinion by discussion, initially between both review authors, and if necessary in consultation with a third review author (RO, JD, or PW). We used the following criteria for selection of studies.
Is it a RCT?
Does at least one arm of the trial involve primarily psychological therapy?
Is the primary aim to reduce pain or to reduce disability associated with pain?
Does the study refer to young persons or adults aged 12 years or older with a presenting problem of painful TMD?
Data extraction and management
We developed a standardised proforma for data extraction and piloted it on a sample of studies for clarity and completeness. We reported outcomes where available immediately postintervention and at long‐term follow‐up. The first review author (CP) and at least one other review author (CB or GT) independently extracted data for all included studies using the standardised proforma. Discrepancies were resolved by discussion and referred to a third review author (RO, JD, or PW) in cases where we could not reach agreement. Relevant studies were translated if not written in English. We included multiple reports of the same study when these were available and attempted to clarify with study authors where this was not clear. We recorded relevant information about the characteristics and findings from all included studies, using a proforma to record:
basic study characteristics, such as study title and type;
inclusion and exclusion criteria;
participant characteristics and demographic details;
study methods including study design, sampling methods, sample size, method of random sequence generation, and any attempt at blinding;
intervention details, such as type, content, home practice expectations, provider, delivery format, and number, length and frequency of sessions;
outcome data, such as outcome measure type, tool, units, and frequency and timing of outcome measurement; any outcomes not prespecified; adverse events;
number of participants randomised and analysed;
number of withdrawals, exclusions, loss to follow‐up;
data analysis method, attrition, and dispersion/precision;
source of funding;
ethical approval and consent.
Assessment of risk of bias in included studies
We applied the Cochrane RoB 1 tool to included studies (Higgins 2017). Three review authors (CP, CB, GT) independently assessed each included study for any risk of bias in sequence generation, allocation concealment, blinding of outcome assessors, incomplete outcome data, selective reporting of outcomes, and other issues. Blinding of participants and staff for this kind of trial is not possible, since people know what intervention they have delivered or attended. The review authors assessed the risk of bias for each study as either low, unclear, or high; they resolved discrepancies by discussion between themselves. We illustrated risk of bias ratings with quotes and information from the primary research papers in each domain.
Measures of treatment effect
We reported data about measures of treatment effect immediately after treatment and at follow‐up. We had planned to calculate the risk ratio (RR) and corresponding 95% confidence interval (95% CI) for any dichotomous outcomes, but none of the studies reported dichotomous outcomes. We calculated the mean difference (MD) and corresponding 95% CI for continuous outcomes that had been measured using the same units, and standardised mean differences (SMD) with corresponding 95% CI for continuous outcomes where different scales had been used to evaluate the same outcome. We applied criteria specified by Cohen 1983 for reporting effect sizes: 0.2 represents a small effect, 0.5 a moderate effect, and 0.8 a large effect. For the primary outcome of pain, we reported whether or not a clinically meaningful reduction in pain was achieved for each study. We did this by calculating within‐group differences from baseline to treatment completion and to follow‐up and comparing these to estimates of clinically‐meaningful pain reduction, representing approximately 30% from baseline, as described in previous reviews of pain outcome measures (Al‐Baghdadi 2014; Dworkin 2005; Farrar 2001; Smith 2020).
Unit of analysis issues
Cluster‐randomised trials
We did not include any cluster‐randomised trials. We had planned to consider data from any cluster‐randomised trials at the same level as the allocation, using a summary of data from each cluster as the primary data and considering the sample size to be the number of clusters and proceed as if the trial were individually randomised, following guidance in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2019a).
Cross‐over trials
We did not find data from cross‐over trials. Our plan was to include data from cross‐over trials separately for each phase of the trial if results were reported before and after the cross‐over, and otherwise to report outcomes narratively.
Studies with multiple treatment groups
In studies which included more than one treatment arm defined as psychological therapy, we included both in the analyses. In order to avoid double counting of participants in the alternative or control groups, we split the relevant treatment alternatives into two halves and considered each intervention group against each half of the alternative treatment or control group.
Dealing with missing data
We extracted data on the basis of intention‐to‐treat. We contacted study authors to ask for missing data such as standard deviations (SDs) if the studies were less than 10 years old. In instances where we were unable to access this information, we described the studies as part of a narrative review only.
Assessment of heterogeneity
The nature of the interventions is such that a high degree of both clinical and methodological heterogeneity would be expected. We were not able to separately analyse interventions based on clinical characteristics. We therefore grouped studies together and described clinical characteristics of studies alongside the meta‐analysis in order to provide a context for the results. For studies that we combined in meta‐analyses, we assessed heterogeneity of treatment effects by visual inspection of forest plots and by using the Chi² test (with a significance level at P < 0.10) and the I² statistic. We based our interpretation of the I² results on that suggested by Higgins 2019b: 0% to 40% might not be important; 30% to 60% may represent moderate heterogeneity; 50% to 90% may represent substantial heterogeneity; and 75% to 100% may represent very substantial (‘considerable') heterogeneity.
Assessment of reporting biases
We searched trial registries and contacted authors of published studies included in the review to ask if they were aware of unpublished research that might be relevant to the review, in order to reduce potential publication bias. There were insufficient studies included in the meta‐analyses to conduct funnel plot analyses.
Data synthesis
We combined data where we identified more than one study reporting an outcome. We did this regardless of statistical heterogeneity (as measured by the I² statistic) and made a note of statistical heterogeneity to put the results in context. We inputted data into Review Manager 5 (Review Manager 2020), and we conducted separate analyses at immediate post‐treatment and longest available follow‐up, using a random‐effects model. We recorded the effect of psychological therapies on pain, disability and psychological distress. We planned to also do so for the outcomes of additional physical symptoms and quality of life; however we did not synthesise data for the latter two outcomes due to insufficient primary studies reporting on these outcomes.
Subgroup analysis and investigation of heterogeneity
If sufficient data were available, we planned to conduct subgroup analyses by age (adolescent versus adult), disability (low versus high) and time since TMD onset (> 3 months and ≤ 12 months versus ≥ 12 months). We defined ‘high disability' as high risk of poor outcome or high severity where the rationale was explained and based on a standardised protocol in the original paper. This type of stratification in TMD studies has typically used scores based on the Graded Chronic Pain Scale (Von Korff 1992), or the Multidimensional Pain Inventory (Okifuji 1999). We planned to consider studies of participants aged 18 years and over against those whose participants were aged 12 to 17 years; data from studies is often restricted to adults, although the onset of TMD frequently occurs during adolescence (Christidis 2019). Where we were unable to combine studies, we reported findings narratively.
Sensitivity analysis
We performed a sensitivity analysis by separately reporting data from studies in which diagnosis was based on established reference standard diagnostic criteria, for example, RDC/TMD (Dworkin 1992), or DC/TMD guidelines (Schiffman 2014).
Summary of findings and assessment of the certainty of the evidence
We used the GRADE approach to assess the certainty of the evidence related to all outcomes listed in the types of outcome measures section above (Schünemann 2017). Using GRADEpro GDT (GRADEpro GDT), two review authors (CP, JD) assessed the certainty of the evidence as ‘high', ‘moderate', ‘low', or ‘very low' depending on the presence and extent of five factors: risk of bias, inconsistency of effect, indirectness, imprecision, and publication bias. We prepared two summary of findings tables for CBT comparisons. Data for other psychological approaches, including behavioural therapy, were insufficient to be collated in any meaningful way. We reported outcomes at treatment completion and at the longest available follow‐up. For each comparison, we reported data on pain, disability, adverse events, and psychological distress, where available. We noted the total number of sessions with an appropriately trained professional and the number of weeks of active psychological treatment where this information was reported.
Results
Description of studies
Results of the search
The study flow diagram is shown in Figure 1. Our searches found 2822 records, which we imported for screening. We identified one additional reference through reference lists of relevant studies. After removing duplicates, we had 1785 records. We rejected 1717 records from a screen of titles and abstracts. We read the full text of 68 papers. We rejected 10 of these because they were not RCTs; five were relevant ongoing studies (see Characteristics of ongoing studies), and we excluded 20 with reasons presented in Characteristics of excluded studies. This left 22 studies, described in 33 separate papers, for inclusion.
1.
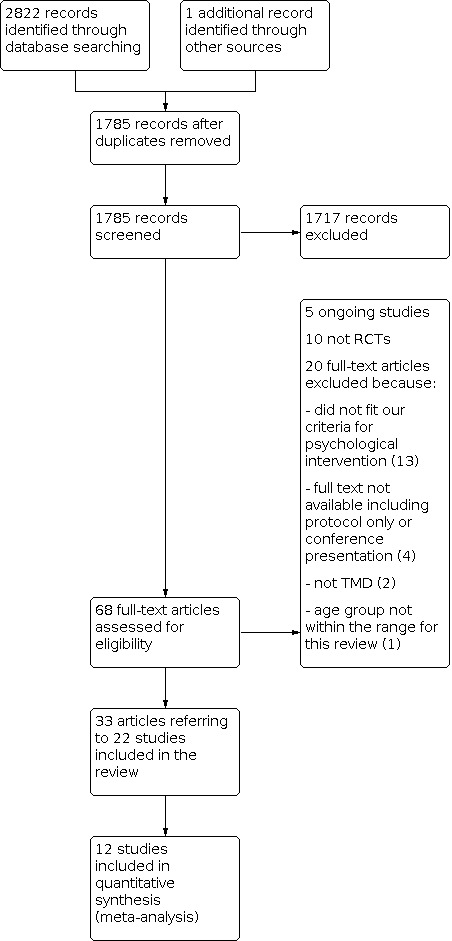
Study flow diagram
Included studies
We included 33 papers in this review, which described 22 RCTs with a total of 2001 participants (ranging from 24 to 191 per trial). The studies were clinically varied, with different numbers and durations of treatment sessions, models of treatment, home practice expectations, inclusion criteria, and outcomes (see Characteristics of included studies).
Design
Twenty‐one RCTs were parallel arm in design; one study was a cross‐over trial but functioned as a parallel study in this review as we used first‐period data only (Wahlund 2015). Eight studies were multi‐arm trials: five with 3 arms (Lupton 1968; Stam 1984; Turk 1993; Turner 2011; Wahlund 2003) and three with 4 arms (Calderon 2011; Mishra 2000; NCT00066937). We did not use one of the arms from Wahlund 2003 (control ‐ brief information).
Setting
All but one of the studies took place in a specialist or university clinic (Townsend 2001). Studies were conducted in the USA (Bartley 2019; Dworkin 1994; Dworkin 2002a; Dworkin 2002b; Gatchel 2006; Litt 2010; Lupton 1968; Mishra 2000; Turk 1993; Turk 1996; Townsend 2001; Turner 2006; Turner 2011), Sweden (Wahlund 2003; Wahlund 2015), Brazil (Calderon 2011), Canada (Stam 1984), Denmark (Abrahamsen 2011), Germany (Mora 2013), and Russia (Shevtsova 2020). Eleven studies were funded by the American National Institutes of Health (NIH), six were unfunded, one was funded by the American Pain Society, and four had other national or local funders.
Participants
All but two studies involved adult participants. Two studies were carried out with adolescents aged 12 to 19 years (Wahlund 2003; Wahlund 2015). These studies compared a formal relaxation programme delivered by a trained therapist versus use of an occlusal appliance. We were unable to include these studies in the data synthesis due to the format in which the outcomes were reported.
Most of the studies included a mix of people who had experienced persistent pain related to TMD for at least 6 months, and often over many years. One study specifically recruited people with recent onset of pain (within the past year) (Gatchel 2006). Two others used an algorithm to allocate participants who were deemed high or low complexity to different interventions (Dworkin 2002a; Dworkin 2002b).
Psychological interventions
The majority of the studies evaluated CBT (alone or in combination with biofeedback) (Calderon 2011; Dworkin 2002a; Dworkin 2002b; Dworkin 1994; Gatchel 2006; Litt 2010; Mishra 2000; Mora 2013; NCT00066937; Turk 1993; Turk 1996; Turner 2006; Turner 2011), with the remainder evaluating CBT and hypnosis in a single treatment package (Ferrando 2012), relaxation (Abrahamsen 2011; Stam 1984; Wahlund 2003; Wahlund 2015), hypnosis (Abrahamsen 2011; Stam 1984), habit reversal training (Townsend 2001), a 'hope‐based intervention' (Bartley 2019), mindfulness (Shevtsova 2020), and education and counselling (Lupton 1968).
Comparison interventions
Alternative treatment
Pharmacological interventions (either alone or in combination with psychological intervention) (Calderon 2011; NCT00066937; Shevtsova 2020; Turner 2011)
Occlusal appliances (either alone or in combination with psychological intervention) (Mora 2013; Turk 1993; Wahlund 2003; Wahlund 2015)
Self‐care management (Turner 2006)
Disease management (alone or with medication) (NCT00066937)
Pain education (Bartley 2019)
Non‐directive counselling (Turk 1996)
Another psychological intervention (or combination): hypnosis versus relaxation (Abrahamsen 2011; Stam 1984); CBT versus biofeedback or combination of CBT + biofeedback (Mishra 2000).
Usual care or no treatment
Usual care (Dworkin 1994; Dworkin 2002a; Dworkin 2002b; Ferrando 2012 ; Gatchel 2006; Litt 2010; Lupton 1968)
Waiting‐list control/no treatment/placebo (Calderon 2011; Mishra 2000; Stam 1984; Townsend 2001; Turk 1993)
Outcomes
Primary outcomes
We selected one measure from each study for each of the outcomes of interest. Where a study measured a single outcome using more than one measure, we selected the measure to include based on IMMPACT recommendations for measuring chronic pain (Dworkin 2005). For pain intensity, we included pain reported on a measure that included combined numerical rating scales, such as the Characteristic Pain Intensity (CPI) (Von Korff 1992) or the Brief Pain Inventory (Tan 2004), where available. Pain intensity was reported in this way in 12 studies (Abrahamsen 2011; Dworkin 1994; Dworkin 2002a; Dworkin 2002b; Ferrando 2012; Mishra 2000; Gatchel 2006; Mora 2013; Turner 2006; Turner 2011; Wahlund 2003; Wahlund 2015). Where combined numerical rating measures were not used, we chose pain intensity rated by a single numerical rating scale (NCT00066937), a visual analogue scale (Calderon 2011) or other psychometrically validated pain measures such as the McGill Pain Questionnaire (Melzack 1975), which was reported by Turk 1996. Where none of these measures were available, we used reports of a subscale of a validated pain questionnaire, as reported by Litt 2010 and Townsend 2001.
For pain disability, we prioritised validated pain disability questionnaires including the Oral Health Impact Profile used by Calderon 2011, Pain Disability Index reported by Mora 2013, followed by validated pain interference questionnaires, which included the Chronic Pain Self‐efficacy Scale reported by Litt 2010 and the Pain Self‐Efficacy Questionnaire reported by Bartley 2019. If none of these scales were reported, we used the overall category from the Graded Chronic Pain Scale as reported by Dworkin 2002a, Mishra 2000 and Turner 2006, or a subscale of a validated pain scale as reported by Turk 1996 and Ferrando 2012.
We reported adverse events in any way that was described by study authors.
We reported outcomes at the end of treatment (shortest duration 4 weeks) and longest post‐treatment follow‐up (12 months).
Secondary outcomes
For psychological distress, we used validated depression questionnaires: the Center for Epidemiological Studies for Depression (CES‐D, Radloff 1977), reported by Bartley 2019, Mora 2013, Litt 2010; Beck Depression Inventory‐II (BDI‐II, Beck 1988) reported by Calderon 2011, Gatchel 2006, Turk 1996, Turner 2006, Turner 2011; or the Short‐Form‐36 (SF‐36, Ware 1994) used by NCT00066937. Otherwise we used an anxiety scale (no studies reported anxiety in the absence of depression), or a relevant subscale of a validated instrument (Abrahamsen 2011; Dworkin 1994; Ferrando 2012).
We reported whichever measure was used to document additional physical symptoms and quality of life: screening for somatoform symptoms (Mora 2013), or relevant subscales from Profile of Mood States (POMS, McNair 1971 used by Mishra 2000) or Symptom Checklist‐Revised (SCL‐90‐R, Derogatis 1983) reported by Dworkin 1994, Dworkin 2002a and Dworkin 2002b.
Available data
Of the studies reporting outcomes of CBT, 12 included quantitative data that could be combined (Dworkin 2002a; Dworkin 2002b; Dworkin 1994; Gatchel 2006; Litt 2010; Mishra 2000; Mora 2013; NCT00066937; Turk 1993; Turk 1996; Turner 2006; Turner 2011), and one presented data that did not include data suitable for meta‐analysis (Calderon 2011). As mentioned above, one study combined CBT and hypnosis in a single package (Ferrando 2012). Of the remaining eight studies, four included data that could be quantitatively analysed (Abrahamsen 2011; Bartley 2019; Townsend 2001; Wahlund 2015), and four could not be combined (Lupton 1968; Shevtsova 2020; Stam 1984; Wahlund 2003).
Excluded studies
We excluded 20 RCTs, described in 20 separate papers after looking at the full texts (see Characteristics of excluded studies). Our reasons for exclusion were that they did not fit our criteria for psychological intervention (13 studies); participants were not diagnosed with persistent TMD (2 studies); data of participants with TMD could not be disaggregated (1 study); or there was insufficient information for inclusion as they were conference proceedings only or we could not source the full text (4 studies).
Ongoing studies
Five studies are ongoing and may be included in the update of this review if data are available by then (see Characteristics of ongoing studies).
Risk of bias in included studies
Our risk of bias judgements are shown in Figure 2. We followed the six Cochrane categories of random sequence generation, allocation concealment, blinding, incomplete outcome data, selective reporting and other potential sources of bias.
2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study
Allocation
We assessed that 11 studies provided a clear description of randomisation and were at low risk of bias for random sequence generation. One study was at unclear risk of bias, and we judged that 10 were at high risk of bias.
Only seven studies reported allocation concealment, and we assessed these as being at low risk of bias for this domain. We assessed that three studies were unclear and 12 were at high risk of bias.
Overall, we judged seven studies to be at low risk of selection bias (Calderon 2011; Ferrando 2012; Mora 2013; Shevtsova 2020; Turner 2006; Turner 2011; Wahlund 2015), two to be unclear (Abrahamsen 2011; NCT00066937), and the remaining 13 to be at high risk.
Blinding
Blinding of participants and personnel is not possible with psychological interventions since both those providing and receiving the intervention must necessarily know what they are providing or receiving.
Eight studies provided information about blinding of outcome assessors that led us to rating them as low risk of detection bias (Bartley 2019; Dworkin 1994; Ferrando 2012; Mora 2013; Shevtsova 2020; Stam 1984; Wahlund 2003; Wahlund 2015), one as unclear (NCT00066937), and the remainder at high risk of detection bias.
Incomplete outcome data
We judged 12 studies as low risk of bias for incomplete outcome data (Bartley 2019; Dworkin 1994; Dworkin 2002a; Gatchel 2006; Litt 2010; Mora 2013; Shevtsova 2020; Turk 1993; Turk 1996; Turner 2006; Turner 2011; Wahlund 2015). These studies reported rates of dropout that were low and used intention‐to‐treat analyses. We judged an unclear risk of bias for one study (Abrahamsen 2011), and high risk of attrition bias for the remaining nine.
Selective reporting
We based our judgement on whether all outcomes listed in the methods section were reported and whether there was a plausible rationale for including these outcomes. On this basis, we judged 16 studies to be at low risk of reporting bias, three studies to be unclear (Dworkin 2002b; Shevtsova 2020; Turner 2011), and three studies to be at high risk of reporting bias (Mishra 2000; NCT00066937; Stam 1984).
Other potential sources of bias
We judged that 17 studies had no other potential sources of bias that we could identify, two were unclear (Gatchel 2006; Shevtsova 2020), and the remaining three were at high risk of bias. Of these three, two were conducted over 30 years ago (Lupton 1968; Stam 1984), and the other was reported in a clinical trials registry but never published (NCT00066937).
Effects of interventions
CBT versus alternative treatment
Six studies reported results of comparisons of CBT against alternative treatments on at least one outcome that we were able to combine (Mora 2013; NCT00066937; Turk 1993; Turk 1996; Turner 2006; Turner 2011). Alternative treatments were diverse and included an intraoral appliance (Mora 2013; Turk 1993), self‐care management (Turner 2006), disease management plus placebo (NCT00066937), disease management plus nortriptyline (NCT00066937), and oral contraceptive management (Turner 2011). See Table 1 for key results for this comparison.
Primary outcomes
Pain intensity
Pain intensity at treatment completion was reported by five studies of 509 participants (Mora 2013; NCT00066937; Turk 1993; Turner 2006; Turner 2011). There was no evidence of any difference between CBT and alternative treatments at treatment completion (standardised mean difference (SMD) 0.03, confidence interval (CI) ‐0.21 to 0.28; heterogeneity measured by I² = 44%; low‐certainty evidence; Analysis 1.1). At longest available follow‐up (between 6 and 12 months), there was a small difference in favour of CBT for pain intensity (SMD ‐0.29, CI ‐0.50 to ‐0.08; I² = 17%; 5 studies, 475 participants; low‐certainty evidence; Analysis 1.2). One study presented data that could not be combined to compare CBT with an alternative treatment (Calderon 2011). Findings were inconsistent with regard to interventions and alternative treatment groups achieving at least a 30% reduction in pain intensity at treatment completion and at follow‐up (Table 3).
1.1. Analysis.

Comparison 1: CBT versus alternative active intervention, Outcome 1: Pain intensity at treatment completion
1.2. Analysis.

Comparison 1: CBT versus alternative active intervention, Outcome 2: Pain intensity at follow‐up
1. Pain reduction from baseline (30% reduction threshold).
| Study | Intervention | Comparison | At treatment completion | At follow‐up | ||
| Intervention | Control | Intervention | Control | |||
| Abrahamsen 2011 | Hypnosis | Relaxation | ✓ 36% |
✕ 7% |
‐ | ‐ |
| Bartley 2019 | Hope based | Pain education | ✕ 15% |
✕ 13% |
‐ | ‐ |
| Calderon 20111,2 | CBT + placebo | Placebo | ✓ 73% |
✓ 45% |
✓ 49% |
✓ 32% |
| Dworkin 1994 | CBT | Usual care | ✕ 28% |
✓ 31% |
✓ 47% |
✓ 33% |
| Dworkin 2002a 1 | CBT | Usual care | ✓ 38% |
✕ 18% |
✓ 40% |
✓ 34% |
| Dworkin 2002b1 | CBT | Usual care | ✓ 36% |
✓ 31% |
✓ 51% |
✓ 33% |
| Ferrando 2012 | CBT + hypnosis | Usual care | ✓ 51% |
✕ 16% |
✓ 55% |
✓ |
| Gatchel 2006 | CBT/biofeedback | Usual care | ‐ | ‐ | ✓ 62% |
✓ 42% |
| NCT000669373 | CBT + placebo | Disease management + placebo | ✓ 39% |
✓ 44% |
✓ 55% |
✓ 56% |
| Disease management + nortriptyline | ✓ 56% |
✓ 69% |
||||
| Litt 20104 | CBT | Usual care | ✕ | ✕ | ✕ | ✕ |
| Lupton 1968 | Education | Usual care | ‐ | ‐ | ‐ | ‐ |
| Counselling | ‐ | ‐ | ‐ | ‐ | ||
| Mishra 20005 | CBT | Waiting‐list control | ✕ 23% |
✕ 11% |
✓ 33% |
✕ 21% |
| CBT/biofeedback | ✕ 26% |
✓ 51% |
||||
| Mora 2013 | CBT/biofeedback | Intraoral appliance | ✕ 28% |
✓ 30% |
✓ 33% |
✕ 24% |
| Shevtsova 2020 | Mindfulness + medication | Medication alone | ✓ | ✓ | ✓ | ✓ |
| Stam 19846 | Hypnosis | Relaxation | ✕ 27% |
✓ 31% |
‐ | ‐ |
| Townsend 2001 | Habit reversal | Waiting‐list control | ✓ 46% |
✕ ‐6% |
✓ 58% |
‐ |
| Turk 19937 | CBT | Intraoral appliance | ✕ 29% |
✓ 54% |
✓ 41% |
✓ 34% |
| Turk 19968 | CBT | Non‐directive counselling | ‐ | ‐ | ‐ | ‐ |
| Turner 2006 | CBT | Self‐care management | ✕ 24% |
✕ 24% |
✓ 42% |
✓ 31% |
| Turner 2011 | CBT/self‐care management | Oral contraceptive | ✓ 38% |
✓ 32% |
✓ 44% |
✕ 26% |
| CBT/targeted | ✓ 42% |
✓ 44% |
||||
| Wahlund 20031,9 | Relaxation | Oral appliance | ✕ | ✓ | ✕ | ✓ |
| Wahlund 2015 | Relaxation | Oral appliance | ✕ 22% |
✓ 37% |
‐ | ‐ |
✓ = ≥ 30% reduction in pain from baseline ✕ = < 30% reduction in pain from baseline
CBT: cognitive behaviour therapy
1Data not suitable for meta‐analysis. 2Other treatment arms (not presented here): CBT plus amitriptyline, amitriptyline alone. 3Other treatment arm (not presented here): CBT + nortriptyline. Benztropine used as placebo. 4Baseline data not presented; unable to calculate % reduction. 5Biofeedback only group (not presented here) also evaluated but the difference between the CBT and biofeedback groups was not clear. 6Waiting‐list control arm (not presented here) showed an increase in pain. 7Waiting‐list control also evaluated but data only available until end of treatment. 8Both groups also received splint. 9Both groups also received behavioural intervention.
Sensitivity analysis
We removed studies that had not used an established diagnostic method, such as the RDC/TMD to diagnose TMDs. This left only two studies that evaluated pain intensity at treatment completion (Turner 2006; Turner 2011) (SMD ‐0.09, 95% CI ‐0.33 to 0.15; I² = 0%; 276 participants; Analysis 1.3). Pain intensity at follow‐up was reported by two studies, with data for 290 participants in total, with a SMD of ‐0.45 (95% CI ‐0.69 to ‐0.21; I² = 0%; Analysis 1.4).
1.3. Analysis.

Comparison 1: CBT versus alternative active intervention, Outcome 3: Pain intensity at completion (sensitivity analysis structured diagnosis only)
1.4. Analysis.

Comparison 1: CBT versus alternative active intervention, Outcome 4: Pain intensity at follow‐up (sensitivity analysis structured diagnosis only)
Disability caused by pain
Three studies representing a total of 245 participants reported data on pain disability. Heterogeneity for this outcome was 0% at both treatment completion and follow‐up. There was no evidence of any difference between CBT and alternative treatment at treatment completion (SMD ‐0.15, CI ‐0.40 to 0.10; I² = 0%; low‐certainty evidence; Analysis 1.5) or at follow‐up (SMD ‐0.15, CI ‐0.42 to 0.12; I² = 0%; low‐certainty evidence; Analysis 1.6).
1.5. Analysis.

Comparison 1: CBT versus alternative active intervention, Outcome 5: Pain disability at treatment completion
1.6. Analysis.

Comparison 1: CBT versus alternative active intervention, Outcome 6: Pain disability at follow‐up
Adverse events
Adverse effects were reported by three studies. NCT00066937 reported minor adverse effects including constipation, dry mouth or sedation in 56% to 78% of participants, all of whom took nortriptyline or benztropine. Turner 2011 reported that no adverse effects were recorded in their self‐management intervention and that breakthrough bleeding (47%), increased appetite or weight gain (11%), increased moodiness (11%), breast tenderness (8%), and increased acne (8%) were relatively common occurrences in those treated with a combined oral contraceptive. Mora 2013 reported that 7 out of 27 people treated with an oral splint reported worse symptoms compared with 3 out of 29 people in the biofeedback with CBT group.
Secondary outcomes
Psychological distress
Psychological distress at treatment completion was reported by six studies of 553 participants. There was evidence of a small advantage of CBT over alternative treatments on this outcome (SMD ‐0.32, CI ‐0.50 to ‐0.15; I² = 3%; low‐certainty evidence; Analysis 1.7). At follow‐up, including 516 participants from 6 studies, the small advantage of CBT was maintained (SMD ‐0.32, CI ‐0.51 to ‐0.13; I² = 8%; low‐certainty evidence; Analysis 1.8).
1.7. Analysis.

Comparison 1: CBT versus alternative active intervention, Outcome 7: Psychological distress at treatment completion
1.8. Analysis.

Comparison 1: CBT versus alternative active intervention, Outcome 8: Psychological distress at follow‐up
Additional physical symptoms
This outcome was not measured.
Quality of life
This outcome was not measured.
CBT versus usual care or waiting list/no treatment control
We combined usual care, no‐treatment control and waiting‐list control, as conditions that did not include any active alternative treatments mimicked usual clinical care, and did not require usual treatment to be specifically withheld for the duration of the trial. Seven studies included data that could be combined for at least one outcome (Dworkin 2002a; Dworkin 2002b; Dworkin 1994; Gatchel 2006 Litt 2010; Mishra 2000; Turk 1993). An additional study evaluated CBT plus placebo against placebo alone but did not include data suitable for inclusion in a meta‐analysis (Calderon 2011). See Table 2 for key results for this comparison.
Primary outcomes
Pain intensity
For CBT against control, six studies of 577 participants in total reported pain intensity at treatment completion (Dworkin 1994; Dworkin 2002a; Dworkin 2002b; Litt 2010; Mishra 2000; Turk 1993). At treatment completion, I² was 34%, and at follow‐up I² was 38%. There was no evidence of a difference between CBT and control for pain intensity at treatment completion (SMD ‐0.09, CI ‐0.30 to 0.12; P = 0.41; low‐certainty evidence; Analysis 2.1). Six studies of 639 participants reported pain intensity at follow‐up (12 months) (Dworkin 1994; Dworkin 2002a; Dworkin 2002b; Gatchel 2006; Litt 2010; Mishra 2000). The SMD suggested a small effect in favour of CBT (SMD ‐0.30, CI ‐0.51 to ‐0.09; P = 0.004; low‐certainty evidence; Analysis 2.2). At follow‐up, at least 30% pain intensity was reported for CBT in all included studies. Findings were inconsistent within the control groups (Table 3).
2.1. Analysis.

Comparison 2: CBT versus control, Outcome 1: Pain intensity at completion
2.2. Analysis.

Comparison 2: CBT versus control, Outcome 2: Pain intensity at follow‐up
Disability caused by pain
Pain disability at treatment completion was reported by three studies of 315 participants. No evidence was found for a difference between CBT and control (SMD 0.02, CI ‐0.21 to 0.24; P = 0.88, I² = 0%; low‐certainty evidence; Analysis 2.3). At follow‐up, two studies of 240 participants reported disability and I² was 83%. No evidence was found for a difference between CBT and control (SMD 0.01, CI ‐0.61 to 0.64; P = 0.97; low‐certainty evidence; Analysis 2.4).
2.3. Analysis.

Comparison 2: CBT versus control, Outcome 3: Pain disability at completion
2.4. Analysis.

Comparison 2: CBT versus control, Outcome 4: Pain disability at follow‐up
Adverse events
Adverse events were reported only by Litt 2010, who reported worsening symptoms in 13 people treated with an oral splint and four treated with CBT.
Secondary outcomes
Psychological distress
Psychological distress was only reported by Litt 2010. No evidence of a difference was found between CBT and standard treatment at completion (MD 2.36, 95% CI ‐1.17 to 5.89; P = 0.19; low‐certainty evidence; Analysis 2.5), or at follow‐up (MD ‐1.02, 95% CI ‐4.02 to 1.98; P = 0.51; low‐certainty evidence; Analysis 2.6).
2.5. Analysis.

Comparison 2: CBT versus control, Outcome 5: Psychological distress at completion
2.6. Analysis.

Comparison 2: CBT versus control, Outcome 6: Psychological distress at follow‐up
Additional physical symptoms
This outcome was not measured.
Quality of life
This outcome was not measured.
GRADE assessment
We downgraded the certainty of the evidence by two levels for all outcomes other than adverse events, based on imprecision and risk of bias. Therefore our confidence that the results represent a true effect is low. We downgraded reported results on adverse events by three levels, resulting in a judgement of very low confidence in the results due to inconsistency, imprecision and limitations in study quality (high risk of bias across many of the domains assessed).
Subgroup analyses
Time since TMD onset (> 3 months and ≤ 12 months versus > 12 months) and extent of TMD disability
We found insufficient data to conduct our planned subgroup analyses based on TMD of less than 12 months versus TMD of more than 12 months, or of low versus high disability.
Gatchel 2006 reported promising results from a study of people reporting painful TMD for 12 months or less and who were classed as having a 'high risk' of disabiliity using a predictive algorithm developed by the study authors. These participants were randomised into an early intervention group, receiving six sessions of CBT and biofeedback, or a non‐intervention group. They reported that, after a year, participants of the early intervention group reported significantly less pain and emotional distress than the non‐intervention group. Moreover, while the early intervention group had improved on these measures, the non‐intervention group had become worse.
For those with a longer history of painful TMD, some studies reported differential outcomes depending on various psychological factors measured at baseline. Dworkin 2002a reported significant improvements in pain intensity, activity interference and somatisation from a three session 'self‐care' intervention delivered by trained dental hygienists for people graded as having low disability on the Graded Chronic Pain Scale. For those graded as having high disability, a more intensive psychological intervention led by a psychologist and dentist resulted in immediate improvements in pain intensity and activity interference that were not maintained at 1‐year follow‐up.
Participant age (12 to 17 years versus 18 years and above)
Of the studies included in the review, 20 referred to an adult population (age 18 years or older). Two studies reported outcomes with adolescents. The first, Wahlund 2003, reported that adolescents who received brief information plus an occlusal appliance reported significantly lower pain intensity at treatment completion than those who received brief information and relaxation training. Of those adolescents in the occlusal appliance group, 60% reported at least a 50% reduction in pain by the end of treatment compared to 32% of those in the brief information and relaxation group. The trial authors thought the relatively weak results from relaxation training may be related to the low number of sessions offered (4 individual sessions). A further study by Wahlund 2015 compared an occlusal appliance to eight individual relaxation sessions of 45 minutes each plus instructions for 15 minutes daily home practice. At treatment completion, 62.1% of adolescents in the occlusal appliance group reported that they were completely well or very much improved compared to 17.9% in the relaxation group. At six months, 79.2% of those in the occlusal appliance group and 60% in the relaxation group reported being completely well or very much improved. We did not find any studies reporting on other psychological therapies for people in the adolescent age group.
Other psychological therapies versus control (no treatment, waiting list or usual care)
The clinical heterogeneity of other psychological therapies and comparisons meant that it was impractical to combine them in meta‐analysis as planned. This was due not only to the variety of interventions described, but due to some elements such as relaxation or education being included in some studies as part of the intervention and in others as comparisons. We looked only at what the studies found for pain intensity and adverse events.
Pain intensity
We calculated a clinically significant improvement in pain for each study. We based our calculations on a reduction of at least 30% from baseline of reported pain, in line with published recommendations for estimating clinically‐important pain reduction (Al‐Baghdadi 2014; Dworkin 2005; Farrar 2001; Smith 2020). Data regarding which studies reported at least a 30% pain reduction from baseline are presented in Table 3. Based on this metric, there is limited evidence that psychological treatments, other than CBT, are consistently beneficial in terms of percentage pain reduction measured at treatment completion or follow‐up. It should be noted that these are within‐group comparisons and not a comparison between the intervention and control, therefore breaking the purpose of the randomisation.
Table 4 presents percentage reduction data alongside the mean difference (MD) in pain intensity scores between groups at end of treatment/follow‐up. There remains limited evidence to demonstrate that psychological treatments other than CBT are beneficial in terms of pain intensity reduction. Any evidence of an effect comes from individual trials at high risk of bias.
2. Data on pain intensity presented in studies that could not be combined in meta‐analyses due to treatment heterogeneity.
| Study and comparison | MD from baseline to treatment completion | % change | SD | n | MD (95% CI) |
| Abrahamsen 2011 | |||||
| Hypnosis | 2.9 | 36 | 2.4 | 19 | ‐1.00 (‐2.27 to 0.27) |
| Relaxation | 3.9 | 7 | 1.5 | 19 | |
| Bartley 2019 | |||||
| Hope‐based intervention | 33.5 | 15 | 18.7 | 15 | 0.10 (‐13.52 to 13.72) |
| Pain education | 33.4 | 13 | 18.7 | 14 | |
| Ferrando 2012 | |||||
| CBT/hypnosis | 2.92 | 51 | 2.03 | 30 | ‐2.32 (‐3.52 to ‐1.12) |
| Usual care | 5.24 | 16 | 2.61 | 29 | |
| Stam 1984 | |||||
| Hypnosis | 44.8 | 27 | not reported | 12 | not reported |
| Relaxation | 34.9 | 31 | 15 | ||
| Townsend 2001 | |||||
| Habit‐reversal treatment | 0.96 | 46 | 0.57 | 10 | ‐1.31 (‐1.97 to ‐0.65) |
| Waiting‐list control | 2.27 | 6* | 0.89 | 10 | |
| Wahlund 2003 | |||||
| Relaxation | not reported | 35 | not reported | 41 | not reported |
| Oral appliance | 51 | 42 | |||
| Wahlund 2015 | |||||
| Relaxation | 18.4 | 22 | 9.5 | 28 | 3.70 (‐1.21 to 8.61) |
| Oral appliance | 14.7 | 37 | 9.4 | 29 | |
CBT: cognitive behaviour therapy; MD: mean difference; SD: standard deviation *pain increased from baseline
Adverse events
Two studies of psychological therapies other than CBT reported data on adverse events. Wahlund 2015 reported no adverse treatment effects from treatment with an occlusal appliance or with relaxation therapy. Abrahamsen 2011 reported that one participant who received hypnosis was admitted to a psychiatric hospital during the intervention. According to the study authors, the admission was for reasons unrelated to the study treatment. The other studies did not report whether or not there were adverse events.
Discussion
Summary of main results
We included 22 studies in this review: 13 evaluated CBT; two evaluated relaxation as the experimental condition, and one study evaluated each of mindfulness, hypnosis, mixed CBT and hypnosis, a habit reversal treatment, education and counselling, and a hope‐based intervention. Just over half of the studies used an active comparison, which included oral splints, medications, education and relaxation. The other studies used a usual care or no‐treatment (waiting list) control.
The studies were conducted over a period of more than 40 years. Twelve studies presented data on at least one outcome suitable for meta‐analysis and ten studies reported in a format that could not be included in meta‐analyses. There were high levels of heterogeneity, both in terms of reported results and also study design, comparators, nature and intensity of the psychological treatment and length and severity of symptoms. Using the GRADE approach, we judged our confidence in most effect sizes as low, meaning that reported effect sizes may not represent the true effect size in the wider population.
We carried out an analysis of CBT against alternative treatment and against control. For studies that reported data that could not be included in the meta‐analyses, we reported on whether treatment and alternative or control conditions achieved a minimum reduction in pain intensity of 30%. CBT interventions did not differ from alternative or control conditions for pain intensity at treatment completion, but showed a small advantage over other conditions at follow‐up. There were no differences evident in terms of pain disability between CBT and other alternative or control conditions. Treatment with CBT showed a small advantage in reducing psychological distress at treatment completion and follow‐up compared to alternative treatment. Only one study comparing CBT against control reported psychological distress as an outcome.
Summary of outcomes across comparisons
Pain intensity
There was no evidence of a benefit of CBT against alternative treatment or control at treatment completion for pain intensity. At follow‐up, CBT showed a small advantage compared to alternative treatment and control. There was insufficient evidence to judge whether other psychological therapies had an effect on pain intensity.
Pain disability
There was no evidence of a difference in disability outcomes between CBT and alternative treatments or control at treatment completion or follow‐up. There was insufficient evidence for other forms of psychological treatment.
Psychological distress
CBT showed a small advantage over alternative treatments in terms of distress at treatment completion and follow‐up. There was only one study evaluating CBT against control (usual care), which did not find evidence of a difference between them for psychological distress. There was insufficient evidence to judge the impact of psychological therapies other than CBT on psychological distress in TMD.
Adverse events
Only six studies presented information on adverse events. From the data available, adverse effects associated with psychological treatment tended to be minor and to occur less often than in alternative treatment groups. There was, however, insufficient evidence presented in order to come to a firm conclusion.
Other outcomes
There were insufficient data present on our other outcomes (quality of life and additional physical symptoms) to come to any conclusions. Only one study reported data for each of these outcomes.
Overall completeness and applicability of evidence
Of the studies reported, a relatively high proportion (10 out of 22) included data in a form that could not be combined in meta‐analysis. This was because of the difficulty of grouping heterogenous clinical interventions, or because standard deviations (SDs) or numbers of participants in each group were not reported and we were not able to gain access to this information by writing to the study authors. Studies that did include data that we combined did not tend to report duration or complexity of symptoms prior to treatment. The majority of the studies were carried out in specialist treatment centres and in countries with high levels of health literacy and access to psychological services. Only 11 studies in total reported on pain intensity at follow‐up (5 against alternative treatment, 6 against control). There is, therefore, little evidence available about the long‐term impact of psychological therapy on ongoing symptoms.
It is also of note that three studies (Dworkin 2002a; Dworkin 2002b; Gatchel 2006), selected specific subgroups of people with TMD for inclusion based on clear rationale of who would be more likely to benefit from psychological treatment. Gatchel 2006 included people early in the experience of TMD who were judged to be at 'high risk' of complexity. Dworkin 2002a and Dworkin 2002b separated participants into two groups based on their Graded Chronic Pain Scale classification and provided tailored treatment, which involved a lower intensity treatment for those with low levels of disability and a more intensive treatment for those with higher levels of disability. The outcomes reported in these papers, particularly for people with recent onset pain and lower disability, are positive. Reports from these studies provide preliminary evidence that it may be beneficial to 'match' people to psychological treatments depending on how long they have had pain and on measures of disability. Unfortunately, there is currently not enough evidence available to judge whether such an approach would be effective on a larger scale.
We did not carry out separate analyses as planned of BT and other forms of psychological therapy due to the small numbers (2 studies on hypnosis, 2 on relaxation, 1 on BT, 1 on mindfulness, 1 on education and 1 hope‐based intervention). None of the studies evaluated ACT, compassion‐focused or cognitive functional therapy, even though these are commonly used interventions for persistent pain elsewhere in the body (Penlington 2019b).
Certainty of the evidence
Our certainty in the evidence as a whole was low. We downgraded our confidence in the effects of treatment across most outcomes due to significant imprecision and risk of bias. Adverse events were infrequently reported and our confidence that these were representative was therefore very low. There is, therefore, a high likelihood that further research could change the estimates of effect size reported in this review.
Potential biases in the review process
We aimed to reduce bias by publishing the protocol for this review in advance and by at least two members of the team independently screening the studies, extracting data and assessing risk of bias and evidence certainty. As planned in our study protocol, we only included RCTs where one of the treatment arms involved psychological treatment delivered by qualified psychologists or therapists trained and supervised in the treatment approach specified. We also aimed to reduce bias by selecting outcome measures in advance of finding studies and extracting data. The three main outcomes of pain intensity, pain disability and distress are plausible, meaningful outcomes that are highly likely to be of relevance to this population.
Our database searches (see Appendices) were thorough and inclusive, and included a search of the grey literature; we therefore believe it likely that we have included all relevant studies.
Agreements and disagreements with other studies or reviews
This is the first Cochrane Review of psychological therapies specifically for painful TMD. The findings are broadly consistent with the recent Cochrane Review of 'Psychological therapies for the management of chronic pain (excluding headache) in adults' (Williams 2020). Our findings that CBT was associated with small or very small benefits at treatment completion and follow‐up compared to alternative treatments or usual care are similar to those of Williams 2020. However, we did not find any benefit of psychological treatments on disability outcomes, whereas Williams 2020 did report a small benefit of psychological therapy on this dimension. This could be because Williams 2020 includes more recent trials; the specific targeting of pain disability has been a feature of more recent psychological approaches to managing pain. It could also reflect inherent differences between specific TMD pain and more general chronic pain, or simply a measurement effect. The number and assessed quality of included studies was somewhat lower in our review compared to Williams 2020. We had far less data about approaches other than CBT, but findings of both reviews were still broadly in line for psychological therapies, indicating small benefits of CBT and little to no evidence for psychological therapies other than CBT in this population. Certainly, for this review, this reflects a lack of studies of alternative psychological treatment approaches and this is also broadly in line with Williams 2020.
The findings are also in keeping with a number of reviews of psychological therapies for TMD carried out within the past decade. Aggarwal 2010 reviewed studies of CBT for chronic orofacial pain and reported weak evidence to favour the use of CBT in secondary care, but commented that there was little research into the optimal duration or content of sessions, or to suggest that it could be implemented in primary care settings. Liu 2012 reviewed a selection of studies reporting on the effectiveness of CBT for painful TMD and concluded that the effect of CBT was inconsistent between studies, so no firm conclusions could be drawn. Randhawa 2016 also reviewed a selection of the published studies (those that they considered to be at low risk of bias) and suggested that CBT has similar effects to self‐management for the outcome of pain, but that CBT was superior for pain disability and distress. Although our findings are broadly consistent with all of these reviews, there are some small differences, which can be accounted for by slightly different inclusion criteria. The ability of such slight differences in study selection to influence the findings in this way is an indication of the need for caution in interpreting the results.
Our findings are also consistent with a review by Kotiranta 2014 into tailored treatments for TMD. This review concluded that, where treatments were hypothesised to be suitable for a particular group based on psychosocial or related characteristics of the group, the outcomes of CBT in people with TMD offered this intervention tended to be favourable. While very few trials have taken this approach (and no additional trials since the publication of the review by Kotiranta 2014), we tentatively came to the same conclusion.
Authors' conclusions
Implications for practice.
Consistent with previous reviews, the results of this review show that people who receive psychological treatments (specifically, CBT) for painful TMDs may have slightly less pain and be less distressed at long‐term follow‐up than people who received an alternative treatment or no treatment. However, this difference is small, may not be clinically significant, and for pain intensity is not evident at the point of treatment completion. Our confidence that these results represent a true finding is low, based on characteristics of the evidence that have been reported to date.
The evidence to date is mainly for CBT, and there is very little evidence available about alternative psychological treatment approaches; however, this may not represent a lack of efficacy; there is simply insufficient evidence to make a judgement. The evidence available at the present time does not suggest that CBT or other psychological interventions are, on average, superior to other available interventions.
Little is known about adverse effects that might be associated with psychological treatment. From information that has been reported, psychological treatments may be associated less frequently with adverse effects compared to other available treatments such as medications or oral appliances.
Healthcare professionals who are not psychologists can consider referring people for psychological therapies as a primary management strategy if this is the patient's preference, and they are able to access psychological therapy locally without a long wait.
For psychology professionals, in the absence of evidence about the superiority of one psychological therapy over another, treatment decisions should continue to be based on a careful assessment of each patient, including a formulation of individual factors that may contribute to their ability to engage effectively in self‐management, and regular review of the impact of treatment.
In the absence of further clarity about these findings, none of which are robust, clinicians in practice may consider psychological treatment as a potential intervention for painful TMD. In order to assess the efficacy of psychological therapies for key outcomes, people should be followed up for as long as possible after treatment completion.
Implications for research.
There is a need for further, good‐quality research trials of psychological therapies for painful TMDs. These should cover a range of psychological approaches, including CBT and also other treatment approaches such as BT, ACT and CFT, which are currently under‐researched for TMD. Specific intensities of treatments should be targeted to individual characteristics, with a clear hypothesis of why a certain intervention is thought to be suitable for a certain subset of people. When planning the intervention, researchers should be guided by research into psychological treatments for chronic pain in general, and should not restrict their consideration to research specifically into TMD, which may be limited. Since average effects are small, researchers should consider aiming to identify specific individual characteristics or potential change processes that might address the question of which treatment is most suitable in which situation, rather than aiming to answer a blanket question of which is the best treatment approach.
Data collection should include measures of pain intensity, pain disability and psychological distress, and should continue ideally until at least 12 months post‐treatment. Researchers should be explicit about whether studies are targeting people with TMD in a community, primary care or secondary/tertiary care setting. Since very few studies have been carried out to date in the community or primary care, there is a particular need for high‐quality, pragmatic RCTs in primary care and the community settings that mimic the real‐world conditions in which these treatments might be applied.
There is also a particular need for good‐quality RCTs of psychological therapies for TMD in adolescents, as it is common for symptoms to start at this age. We found only two relevant studies from one group that focused on adolescents, and these used relaxation as an intervention. Researchers should consider planning good‐quality trials of psychological therapies against alternative treatments (such as oral splints) or usual care, and include longer‐term follow‐up data.
History
Protocol first published: Issue 12, 2019
Notes
This review is based on a protocol published in the Cochrane Library in December 2019 (Penlington 2019c).
Acknowledgements
We acknowledge the contribution of Cochrane Oral Health to the production of the protocol and the review, specifically Laura MacDonald, Managing Editor; Anne Littlewood, Information Specialist; Professor Anne‐Marie Glenny, Co‐ordinating Editor; Editors Zipporah Iheozor‐Ejiofor, Dr Jennifer Taylor, and Dr Philip Riley; Claudia Bollig and Annette Bluemle for translations; and copy‐editors Deirdre Walsh, Andrea Takeda and Claire Dooley. We also thank peer reviewers Pauline Adair, Iris Gordon and Ranjana Garg. Thank you to Niki Jones for contributing to the plain language summary.
Appendices
Appendix 1. Cochrane Oral Health’s Trials Register search strategy
Cochrane Oral Health’s Trials Register is available via the Cochrane Register of Studies. For information on how the register is compiled, see oralhealth.cochrane.org/trials.
MESH DESCRIPTOR Craniomandibular Disorders EXPLODE ALL AND INREGISTER
MESH DESCRIPTOR Facial Pain AND INREGISTER
(temporomandibular or "temporo mandibular") AND INREGISTER
(TMJ or TMD or TMJD) AND INREGISTER
((TM or TMJ) near1 (disorder* or dysfunction* or disease*)) AND INREGISTER
((facial or face or orofacial or "oro facial") near2 (pain* or neuralgia)) AND INREGISTER
#1 or #2 or #3 or #4 or #5 or #6 AND INREGISTER
MESH DESCRIPTOR Psychotherapy EXPLODE ALL AND INREGISTER
MESH DESCRIPTOR Adaptation, Psychological AND INREGISTER
MESH DESCRIPTOR Relaxation Therapy AND INREGISTER
((cognitive or cognition) near3 (behav* or treatment* or technique* or therap* or intervention* or restructur* or reapprais*)) AND INREGISTER
(behav* near3 (treatment* or therap* or intervention* or activ* or technique* or modif* or change* or adapt* or condition*)) AND INREGISTER
(accept* near5 commitment) AND INREGISTER
(autogenic near1 (train* or relax*)) AND INREGISTER
(mindful* or awareness or mood*) AND INREGISTER
"conditioning therap*" AND INREGISTER
((adapt* or cope or coping) near3 behavi*) AND INREGISTER
(coping near3 (skill* or strateg*)) AND INREGISTER
(psychotherap* or psychological*) AND INREGISTER
(group* near3 (therap* or psychotherap*)) AND INREGISTER
(talk* near3 (therap* or intervention*)) AND INREGISTER
MESH DESCRIPTOR Counseling AND INREGISTER
counsel* AND INREGISTER
#8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 AND INREGISTER
#24 AND #7 AND INREGISTER
Appendix 2. Cochrane Central Register of Controlled Trials (CENTRAL) search strategy
MESH DESCRIPTOR Craniomandibular Disorders EXPLODE ALL AND CENTRAL:TARGET
MESH DESCRIPTOR Facial Pain AND CENTRAL:TARGET
(temporomandibular or "temporo mandibular") AND CENTRAL:TARGET
(TMJ or TMD or TMJD) AND CENTRAL:TARGET
((TM or TMJ) near1 (disorder* or dysfunction* or disease*)) AND CENTRAL:TARGET
((facial or face or orofacial or "oro facial") near2 (pain* or neuralgia)) AND CENTRAL:TARGET
#1 or #2 or #3 or #4 or #5 or #6 AND CENTRAL:TARGET
MESH DESCRIPTOR Psychotherapy EXPLODE ALL AND CENTRAL:TARGET
MESH DESCRIPTOR Adaptation, Psychological AND CENTRAL:TARGET
MESH DESCRIPTOR Relaxation Therapy AND CENTRAL:TARGET
((cognitive or cognition) near3 (behav* or treatment* or technique* or therap* or intervention* or restructur* or reapprais*)) AND CENTRAL:TARGET
(behav* near3 (treatment* or therap* or intervention* or activ* or technique* or modif* or change* or adapt* or condition*)) AND CENTRAL:TARGET
(accept* near5 commitment) AND CENTRAL:TARGET
(autogenic near1 (train* or relax*)) AND CENTRAL:TARGET
(mindful* or awareness or mood*) AND CENTRAL:TARGET
"conditioning therap*" AND CENTRAL:TARGET
((adapt* or cope or coping) near3 behavi*) AND CENTRAL:TARGET
(coping near3 (skill* or strateg*)) AND CENTRAL:TARGET
(psychotherap* or psychological*) AND CENTRAL:TARGET
(group* near3 (therap* or psychotherap*)) AND CENTRAL:TARGET
(talk* near3 (therap* or intervention*)) AND CENTRAL:TARGET
MESH DESCRIPTOR Counseling AND CENTRAL:TARGET
counsel* AND CENTRAL:TARGET
#8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 AND CENTRAL:TARGET
#24 AND #7 AND CENTRAL:TARGET
Appendix 3. MEDLINE Ovid search strategy
exp craniomandibular disorders/
Facial pain/
(temporomandibular or "temporo mandibular").mp.
(TMJ or TMD or TMJD).ti,ab.
((TM or TMJ) adj (disorder$ or dysfunction$ or disease$)).mp.
((facial or face or orofacial or "oro facial") adj2 (pain$ or neuralgia)).mp.
or/1‐6
exp Psychotherapy/
Adaptation, psychological/
Relaxation therapy/
((cognitive or cognition) adj3 (behav$ or treatment$ or technique$ or therap$ or intervention$ or restructur$ or reapprais$)).mp.
(behav$ adj3 (treatment$ or therap$ or intervention$ or activ$ or technique$ or modif$ or change$ or adapt$ or condition$)).mp.
(accept$ adj5 commitment).mp.
(autogenic adj (train$ or relax$)).mp.
(mindful$ or awareness or mood$).mp.
"conditioning therap$".mp.
((adapt$ or cope or coping) adj3 behavi$).mp.
(coping adj3 (skill$ or strateg$)).mp.
(psychotherap$ or psychological$).mp.
(group$ adj3 (therap$ or psychotherap$)).mp.
(talk$ adj3 (therap$ or intervention$)).mp.
counseling/
counsel$.mp.
or/8‐23
7 and 24
The above subject search will be linked with the highly sensitive search strategy designed by Cochrane for identifying randomised controlled trials and controlled clinical trials (as described in the Cochrane Handbook for Systematic Reviews of Interventions Box 6.4.b (Lefebvre 2020)).
randomized controlled trial.pt.
controlled clinical trial.pt.
randomized.ab.
placebo.ab.
drug therapy.fs.
randomly.ab.
trial.ab.
groups.ab.
or/1‐8
exp animals/ not humans.sh.
9 not 10
Appendix 4. Embase Ovid search strategy
Temporomandibular joint disorder/
Face pain/
(temporomandibular or "temporo mandibular").mp.
(TMJ or TMD or TMJD).ti,ab.
((TM or TMJ) adj (disorder$ or dysfunction$ or disease$)).mp.
((facial or face or orofacial or "oro facial") adj2 (pain$ or neuralgia)).mp.
or/1‐6
exp Psychotherapy/
exp Coping behavior/
((cognitive or cognition) adj3 (behav$ or treatment$ or technique$ or therap$ or intervention$ or restructur$ or reapprais$)).mp.
(behav$ adj3 (treatment$ or therap$ or intervention$ or activ$ or technique$ or modif$ or change$ or adapt$ or condition$)).mp.
(accept$ adj5 commitment).mp.
(autogenic adj (train$ or relax$)).mp.
(mindful$ or awareness or mood$).mp.
"conditioning therap$".mp.
((adapt$ or cope or coping) adj3 behavi$).mp.
(coping adj3 (skill$ or strateg$)).mp.
(psychotherap$ or psychological$).mp.
(group$ adj3 (therap$ or psychotherap$)).mp.
(talk$ adj3 (therap$ or intervention$)).mp.
exp counseling/
counsel$.mp.
or/8‐22
7 and 23
The above subject search was linked with the highly sensitive search strategy designed by Cochrane for identifying randomised controlled trials and controlled clinical trials in Embase (as described in Lefebvre 2020, box 3e).
Randomized controlled trial/
Controlled clinical study/
random$.ti,ab.
randomization/
intermethod comparison/
placebo.ti,ab.
(compare or compared or comparison).ti.
((evaluated or evaluate or evaluating or assessed or assess) and (compare or compared or comparing or comparison)).ab.
(open adj label).ti,ab.
((double or single or doubly or singly) adj (blind or blinded or blindly)).ti,ab.
double blind procedure/
parallel group$1.ti,ab.
(crossover or cross over).ti,ab.
((assign$ or match or matched or allocation) adj5 (alternate or group$1 or intervention$1 or patient$1 or subject$1 or participant$1)).ti,ab.
(assigned or allocated).ti,ab.
(controlled adj7 (study or design or trial)).ti,ab.
(volunteer or volunteers).ti,ab.
human experiment/
trial.ti.
or/1‐19
random$ adj sampl$ adj7 ("cross section$" or questionnaire$1 or survey$ or database$1)).ti,ab. not (comparative study/ or controlled study/ or randomi?ed controlled.ti,ab. or randomly assigned.ti,ab.)
Cross‐sectional study/ not (randomized controlled trial/ or controlled clinical study/ or controlled study/ or randomi?ed controlled.ti,ab. or control group$1.ti,ab.)
(((case adj control$) and random$) not randomi?ed controlled).ti,ab.
(Systematic review not (trial or study)).ti.
(nonrandom$ not random$).ti,ab.
"Random field$".ti,ab.
(random cluster adj3 sampl$).ti,ab.
(review.ab. and review.pt.) not trial.ti.
"we searched".ab. and (review.ti. or review.pt.)
"update review".ab.
(databases adj4 searched).ab.
(rat or rats or mouse or mice or swine or porcine or murine or sheep or lambs or pigs or piglets or rabbit or rabbits or cat or cats or dog or dogs or cattle or bovine or monkey or monkeys or trout or marmoset$1).ti. and animal experiment/
Animal experiment/ not (human experiment/ or human/)
or/21‐33
20 not 34
Appendix 5. PsycINFO Ovid search strategy
PsycINFO Ovid search strategy
Joint disorders/
Jaw/
1 and 2
(temporomandibular or "temporo mandibular").mp.
(TMJ or TMD or TMJD).ti,ab.
((TM or TMJ) adj (disorder$ or dysfunction$ or disease$)).mp.
((facial or face or orofacial or "oro facial") adj2 (pain$ or neuralgia)).mp.
3 or 4 or 5 or 6 or 7
exp Psychotherapy/
exp Coping behavior/
Relaxation therapy/
((cognitive or cognition) adj3 (behav$ or treatment$ or technique$ or therap$ or intervention$ or restructur$ or reapprais$)).mp.
(behav$ adj3 (treatment$ or therap$ or intervention$ or activ$ or technique$ or modif$ or change$ or adapt$ or condition$)).mp.
(accept$ adj5 commitment).mp.
(autogenic adj (train$ or relax$)).mp.
(mindful$ or awareness or mood$).mp.
"conditioning therap$".mp.
((adapt$ or cope or coping) adj3 behavi$).mp.
(coping adj3 (skill$ or strateg$)).mp.
(psychotherap$ or psychological$).mp.
(group$ adj3 (therap$ or psychotherap$)).mp.
(talk$ adj3 (therap$ or intervention$)).mp.
exp counseling/
counsel$.mp.
or/9‐24
8 and 25
The subject search was linked to the Cochrane Oral Health filter for PsycINFO:
exp clinical trials/
(clin$ adj25 trial$).ti,ab.
placebo$.ti,ab.
random$.ti,ab.
((randomised adj controlled adj trial$) or (randomized adj controlled adj trial$)).mp.
(controlled adj clinical adj trial$).mp.
(random adj allocat$).mp.
((singl$ or doubl$ or trebl$ or tripl$) adj25 (blind$ or mask$)).ti,ab.
(control$ adj4 trial$).mp.
(ANIMALS not HUMANS).sh.
or/1‐9
11 not 10
Appendix 6. Trip database search strategy
With all the words: (temporomandibular)
With any of the words: psychological or psychotherapy or cognitive or behavior or behaviour or mindfulness or mood or counselling
Appendix 7. Web of Science Conference Proceedings search strategy
# 20 #18 and #19 # 19 TS=(trial* or random* or placebo*) # 18 #4 and #17 # 17 #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 # 16 TS=counsel* # 15 TS=(talk* AND (therap* or intervention*)) # 14 TS=(group* AND (therap* or psychotherap*)) # 13 TS=(psychotherap* or psychological*) # 12 TS=(coping AND (skill* or strateg*)) # 11 TS=((adapt* or cope or coping) AND behavi*) # 10 TS="conditioning therap*" # 9 TS=(mindful* or awareness or mood*) # 8 TS=(autogenic and (train* or relax*)) # 7 TS=(accept* and commitment) # 6 TS=(behav* AND (treatment* or therap* or intervention* or activ* or technique* or modif* or change* or adapt* or condition*)) # 5 TS=((cognitive or cognition) AND (behav* or treatment* or technique* or therap* or intervention* or restructur* or reapprais*)) # 4 #1 or #2 or #3 # 3 TI=(TMJ or TMD or TJMD) # 2 TS=((face or facial or orofacial or "oro facial") AND (pain or neuralgia)) # 1 TS=(temporomandibular or "temporo mandibular")
Appendix 8. Proquest Disserations and Theses Global search strategy
noft(temporomandibular OR "temporomandibular" OR "facial pain" OR "orofacial pain") AND noft(psychotherap* OR "relaxation therap*" OR psychological* OR cognitiv* OR cognition OR behaviour* OR behavior* OR autogenic OR commitment OR awareness OR mood OR mindful* OR "conditioning therap*" OR adapt* OR cope OR coping OR "group therap*" OR psychotherap* OR counsel*) AND noft(random* or trial* or placebo*)
Appendix 9. Open Grey search strategy
temporomandibular and psycho*
Appendix 10. US National Institutes of Health Ongoing Trials Register (ClinicalTrials.gov) search strategy
Expert search:
temporomandibular AND (psychotherap* OR "relaxation therap*" OR psychological* OR cognitiv* OR cognition OR behaviour* OR behavior* OR autogenic OR commitment OR awareness OR mood OR mindful* OR "conditioning therap*" OR adapt* OR cope OR coping OR "group therap*" OR psychotherap* OR counsel*)
Appendix 11. World Health Organization International Clinical Trials Registry Platform search strategy
(temporomandibular AND psychotherap* OR temporomandibular AND "relaxation therap*" OR temporomandibular AND psychological* OR temporomandibular AND cognitiv* OR temporomandibular AND cognition OR temporomandibular AND behaviour* OR temporomandibular AND behavior* OR temporomandibular AND autogenic OR temporomandibular AND commitment OR temporomandibular AND awareness OR temporomandibular AND mood OR temporomandibular AND mindful* OR temporomandibular AND "conditioning therap*" OR temporomandibular AND adapt* OR temporomandibular AND cope OR temporomandibular AND coping OR temporomandibular AND "group therap*" OR temporomandibular AND psychotherap* OR temporomandibular AND counsel*)
Data and analyses
Comparison 1. CBT versus alternative active intervention.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Pain intensity at treatment completion | 5 | 509 | Std. Mean Difference (IV, Random, 95% CI) | 0.03 [‐0.21, 0.28] |
| 1.2 Pain intensity at follow‐up | 5 | 475 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.29 [‐0.50, ‐0.08] |
| 1.3 Pain intensity at completion (sensitivity analysis structured diagnosis only) | 2 | 276 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.09 [‐0.33, 0.15] |
| 1.4 Pain intensity at follow‐up (sensitivity analysis structured diagnosis only) | 2 | 290 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.45 [‐0.69, ‐0.21] |
| 1.5 Pain disability at treatment completion | 3 | 245 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.15 [‐0.40, 0.10] |
| 1.6 Pain disability at follow‐up | 3 | 245 | Mean Difference (IV, Random, 95% CI) | ‐0.15 [‐0.42, 0.12] |
| 1.7 Psychological distress at treatment completion | 6 | 553 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.32 [‐0.50, ‐0.15] |
| 1.8 Psychological distress at follow‐up | 6 | 516 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.32 [‐0.51, ‐0.13] |
Comparison 2. CBT versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Pain intensity at completion | 6 | 577 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.09 [‐0.30, 0.12] |
| 2.2 Pain intensity at follow‐up | 6 | 639 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.30 [‐0.51, ‐0.09] |
| 2.3 Pain disability at completion | 3 | 315 | Std. Mean Difference (IV, Random, 95% CI) | 0.02 [‐0.21, 0.24] |
| 2.4 Pain disability at follow‐up | 2 | 240 | Std. Mean Difference (IV, Random, 95% CI) | 0.01 [‐0.61, 0.64] |
| 2.5 Psychological distress at completion | 1 | 101 | Mean Difference (IV, Random, 95% CI) | 2.36 [‐1.17, 5.89] |
| 2.6 Psychological distress at follow‐up | 1 | 101 | Mean Difference (IV, Fixed, 95% CI) | ‐1.02 [‐4.02, 1.98] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Abrahamsen 2011.
| Study characteristics | ||
| Methods |
Study design: parallel‐group RCT Number of control groups: 1 Number of intervention groups: 1 |
|
| Participants |
Inclusion criteria: RDC/TMD diagnosis of persistent myofascial pain disorder of over 6 months. Comorbidities and stable doses of pain medication did not preclude inclusion. Exclusion criteria: pacemaker, severe psychiatric illness Pretreatment: largely similar at baseline Number eligible for study: not stated Number of participants: 43 Number randomly assigned to intervention (control): 21 in hypnosis group, 22 in relaxation (control) group Number started treatment in intervention (control): 20 (20) Number completed treatment in intervention (control): 19 (15) Number included in analysis from intervention (control): 20 Comorbidity: not stated Sex: 100% female Ethnicity: not specified Other sample characteristics: n/a |
|
| Interventions |
|
|
| Outcomes |
Used in study but not in review:
|
|
| Identification |
Date of study: 2007 to 2008; specific date not mentioned. Paper published 2011 Sponsorship source: not stated Setting: dental hospital Author name: Randi Abrahamsen Institution: Aarhus University Hospital Email: lbhansen@odont.au.dk (second author Lene Baad Hansen) Address: Department of Clinical Oral Physiology, School of Dentistry, Aarhus University, Vennelyst Boulevard 9, DK‐8000 Aarhus C, Denmark |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer randomisation. Quote: "Patients were randomly assigned (randomisation computer program) to a hypnosis group (n = 21) or a control group with nonhypnotic relaxation as the intervention (n = 22)." |
| Allocation concealment (selection bias) | Unclear risk | Detail not provided about steps taken to ensure group allocation could not be predicted |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants were told they were in an active treatment group and that two treatment groups were being compared against each other. Blinding of clinician is not possible in this type of trial |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Self‐reported outcomes ‐ participants completed their own assessments and were not blind to treatment received |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | States intention‐to‐treat but did not account for 1 participant (of 20) with missing data |
| Selective reporting (reporting bias) | Low risk | Outcomes are reported as planned in the methods section. |
| Other bias | Low risk | None noted |
Bartley 2019.
| Study characteristics | ||
| Methods |
Study design: parallel‐group RCT Number of control groups: 1 Number of intervention groups: 1 |
|
| Participants |
Inclusion criteria: adults recruited from the local community reporting moderate facial pain (3 ± 10) on at least 15 days for the past 3 months. Evaluated in clinic by RDC/TMD examination Exclusion criteria: < 18 or > 65 years of age, use of narcotic analgesics, use of nonsteroidal anti‐inflammatory medications 24 hours before pain testing sessions, cardiovascular, neuroendocrine or neurological disorders, cognitive impairment Pretreatment: no significant differences between groups at baseline Number eligible for study: 35 Number of participants: 33 Number randomly assigned to intervention (control): 16 (17) Number started treatment in intervention (control): 16 (17) Number completed treatment in intervention (control): 15 (14) Number included in analysis from intervention (control): 15 (14) Comorbidity: not discussed Sex: not reported Ethnicity: white 21, black 6, other 2 Other sample characteristics: n/a |
|
| Interventions |
|
|
| Outcomes |
Used in study but not in review:
|
|
| Identification |
Date of study: article published 2019. Unclear when study took place Sponsorship source: American Pain Society Sharon S Keller Grant Country: USA Setting: University of Florida Clinical Research Center Author: Emily J Bartley Institution: University of Florida Email: ebartley@dental.ufl.edu Address: Pain Research and Intervention Center of Excellence, College of Dentistry, University of Florida, 1329 SW 16th St, Suite 5192, Gainesville, FL 32610 |
|
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Method not described "Participants...were randomly assigned by the PI following simple randomisation procedures (accounting for equal distribution of men and women across groups)" |
| Allocation concealment (selection bias) | Unclear risk | Does not say whether principal investigator who performed random allocation was blinded |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not discussed. Not possible for this kind of study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Research assistants were blind to group allocation. Participants were told they were randomised to one of two active interventions. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 29 out of 33 participants completed study |
| Selective reporting (reporting bias) | Low risk | Comprehensive data are presented as described in the methods. |
| Other bias | Low risk | None noted |
Calderon 2011.
| Study characteristics | ||
| Methods |
Study design: parallel‐group RCT Number of control groups: 2 Number of intervention groups: 2 |
|
| Participants |
Inclusion criteria: history of orofacial pain for more than 6 months, pain occurring daily or almost daily for at least the month preceding enrolment, pain of at least moderate severity (i.e. at least 40 mm on a visual scale of 0 to 100 mm), age ranging from 17 to 55 years Exclusion criteria: major neurological or psychiatric disorders, glaucoma, history of intolerance to amitriptyline, pain secondary to trigeminal neuralgia, or pain attributable to other local, well defined condition Pretreatment: no statistically significant differences at baseline between groups Number eligible for study: 60 Number of participants: 47 Number randomly assigned to intervention (control): 23 (24) Number started treatment in intervention (control): 23 (24) Number completed treatment in intervention (control): 17 (20) Number included in analysis from intervention (control): 17 (20) Comorbidity: not specified Sex: all female Ethnicity: not reported Age: 35.4 or 35.6 years (reported differently in abstract and main text) (range 17‐52 years) Other sample characteristics: n/a |
|
| Interventions |
|
|
| Outcomes |
Used in study but not review:
|
|
| Identification |
Date of study: paper published in 2011. Study dates not specified Sponsorship source: CAPES ‐ Brazil Setting: outpatient university based orofacial pain clinic Author: Patricia S Calderon Institution: Universidade Federal do Rio Grande do Norte Country: Brazil Email: patriciascalderon@yahoo.com.br Address: Departamento de Odontologia, Universidade Federal do Rio Grande do Norte, Avenida Salgado Filho, 1787, Lagoa Nova, 59056‐000 Natal, RN, Brasil |
|
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was stratified by pain level and generated using the web site ‘www.randomization.com’ by a researcher unrelated to the rest of the study. |
| Allocation concealment (selection bias) | Low risk | "The randomisation scheme was generated using the web site ‘www.randomization.com’ and the researcher was blind to group distribution. Then, a different person was designated to allocate the patients in their groups, for the medicine distribution and to lead the patients to the CBT." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of participants and personnel not possible for the psychological element of the study. Placebo used to blind to medication and administered by an independent person |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Data from participants who dropped out were not included in the analysis. "9 patients dropped out from the study and 1 was excluded from the study due to adverse events (self‐report visual symptoms after taking the medicine)" |
| Selective reporting (reporting bias) | Low risk | Outcomes reported for all measures described in methods |
| Other bias | Low risk | None noted |
Dworkin 1994.
| Study characteristics | ||
| Methods |
Study design: parallel‐group RCT Number of control groups: 1 Number of intervention groups: 1 |
|
| Participants |
Inclusion criteria: referral for treatment of TMD with a self‐report of facial ache/pain in muscles of mastication; TMJ; pre‐ and intra‐auricular Exclusion criteria: pain attributable to: confirmed migraine or head pain condition (other than tension headache), acute infection, significant disease of teeth, ears, eyes, nose or throat, history of significant or debilitating chronic physical or mental illness, requiring emergency TMD treatment Pretreatment: none Number eligible for study: 395 Number of participants: 185 Number randomly assigned to intervention (control): 95 (90) Number started treatment in intervention (control): 95 (90) Number completed treatment in intervention (control): 66 (73) Number included in analysis from intervention (control): 66 (73) Comorbidity: major conditions excluded, otherwise not stated Sex: 85% female, 15% male Ethnicity: 96% white Other sample characteristics: 81% completed more than high school education |
|
| Interventions |
|
|
| Outcomes |
Used in study but not review:
|
|
| Identification |
Date of study: not reported ‐ paper published 1994 Sponsorship source: NIDR Program Project Grant Country: USA Setting: School of Medicine ‐ TMJ Clinic of Group Health Cooperative of Puget Sound (GHC) or Orofacial Pain and Dysfunction Clinic at the University of Washington School of Dentistry (VW) Author name: Samuel F Dworkin Institution: University of Washington Email: none provided Address: Department of Oral Medicine, School of Medicine, University of Washington, Seattle, WA 98195, USA |
|
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation schedule |
| Allocation concealment (selection bias) | High risk | Allocation concealment not discussed |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible to blind participants to this kind of study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome data collected by dental hygienists who were unaware of group allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Participants who dropped out were asked to complete measures in order to allow for an intention‐to‐treat analysis. |
| Selective reporting (reporting bias) | Low risk | A comprehensive list of outcomes is reported. |
| Other bias | Low risk | None observed |
Dworkin 2002a.
| Study characteristics | ||
| Methods |
Study design: parallel‐group RCT Number of control groups: 1 Number of intervention groups: 1 |
|
| Participants |
Inclusion criteria: self‐reported facial pain or ache in region of TMD muscles, diagnosed TMD according to RDC/TMD criteria GCPS group of 0, 1 or 2a, aged 18 to 70 Exclusion criteria: pain according to confirmed migraine or head pain other than tension headache acute infection of teeth or facial area presence of significant or debilitating physical or mental health condition necessity for urgent TMD treatment Pretreatment: no significant difference between groups at baseline other than higher level of education attained with 91.8% of self‐care compared to 57.7% of usual treatment reporting post‐high school education. Baseline measures adjusted for level of education. Number eligible for study: 196 Number of participants: 124 Number randomly assigned to intervention (control): 61 (63) Number started treatment in intervention (control): 48 (55) Number completed treatment in intervention (control): 43 ‐ 61 randomised, 13 dropped out before any sessions, 5 dropped out after up to 2 sessions (51 ‐ 63 randomised, 8 dropped out before any treatment, 4 dropped out from treatment) Number included in analysis from intervention (control): analyses are on intention‐to‐treat basis where data allows. 90% data from those who started treatment available at follow‐up: of 48 patients who began the self‐care intervention, 5 dropped out, and of the 55 patients who began the usual treatment arm, 4 dropped out. Comorbidity: not stated except for exclusion of significantly debilitating comorbidities Sex: 85 female, 15 male Ethnicity: not stated Other sample characteristics: more of the self care group had education beyond high school level. This was therefore adjusted for in the analyses. |
|
| Interventions |
|
|
| Outcomes |
Used in study but not in review:
|
|
| Identification |
Date of study: published 2002 Sponsorship source: National Institute of Dental and Craniofacial Research Country: USA Setting: specialist orofacial pain clinic connected to a university Comments: comprehensive care Author name: Samuel Dworkin Institution: University of Washington Email: dworkin@u.washington.edu Address: Department of Oral Medicine and Psychiatry and Behavioural Sciences |
|
| Notes | Raw data not available in article | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Method of sequence generation not discussed |
| Allocation concealment (selection bias) | High risk | Allocation concealment not specified |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of participants not possible in this type of intervention. Blinding of study personnel not discussed |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Blinding of outcome assessors not discussed |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data are reported where possible on an intention‐to‐treat basis. |
| Selective reporting (reporting bias) | Low risk | Results are presented from the broad range of outcome measures. |
| Other bias | Low risk | None noted |
Dworkin 2002b.
| Study characteristics | ||
| Methods |
Study design: parallel‐group RCT Number of control groups: 1 Number of intervention groups: 1 |
|
| Participants |
Inclusion criteria: self‐report of facial ache or pain in TMD relevant areas, RDC/TMD based diagnosis, Grade 2b, 3 or 4 on GCPS, attending dentist judges treatment needed Exclusion criteria: pain attributable to confirmed migraine or facial condition other than tension headache, acute infection or other significant disease relevant to the face, debilitating physical or mental health condition, requires urgent treatment for TMD, unable to communicate in English Pretreatment: groups largely similar at baseline Number eligible for study: 186 Number of participants: 117 Number randomly assigned to intervention (control): 59 (58) Number started treatment in intervention (control): not stated Number completed treatment in intervention (control): not stated Number included in analysis from intervention (control): 52 post‐treatment and 56 at 12 months in intervention group. 49 post‐treatment and 51 at 12 months in control. Comorbidity: not mentioned. Major comorbidities excluded Sex: 100 female, 17 male Ethnicity: not stated Other sample characteristics: defined as complex on basis of 2b or higher rank on GCPS |
|
| Interventions |
|
|
| Outcomes |
Used in study but not in review:
|
|
| Identification |
Date of study: published 2002 Sponsorship source: National Institute of Dental and Craniofacial Research Country: USA Setting: specialist orofacial pain clinic connected to a university Author name: Samuel Dworkin Institution: University of Washington Email: dworkin@u.washington.edu Address: Department of Oral Medicine and Psychiatry and Behavioural Sciences |
|
| Notes | Raw data not available in article | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Method of sequence generation not discussed |
| Allocation concealment (selection bias) | High risk | Not discussed |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of participants not possible in this type of intervention Blinding of study personnel not discussed |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not discussed |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Although efforts were made for an intention‐to treat analysis, data were missing from 16 participants at treatment follow‐up and 10 at 1 year follow‐up |
| Selective reporting (reporting bias) | Unclear risk | All measures collected are reported in the text. Most, however, do not include raw data that can be included in meta‐analysis. |
| Other bias | Low risk | None noted |
Ferrando 2012.
| Study characteristics | ||
| Methods |
Study design: parallel‐group RCT Number of control groups: 1 Number of intervention groups: 1 |
|
| Participants |
Inclusion criteria: TMD muscular subgroup diagnosis (group 1 axis I diagnosis) following RDC/TMD2, intellectual ability to follow the evaluation process and psychologic intervention Excluded criteria: abnormalities, such as facial deformity, tumoral pathology, or lesions of the oral mucosa (e.g. erosive lichen planus, pemphigus, pemphigoids, or large aphthae), evidence in medical records of psychotic disorders according to the DSM Pretreatment: no significant differences in group allocation Number eligible for study: 85 Number of participants: 72 Number randomly assigned to intervention (control): 41 (31) Number started treatment in intervention (control): 41 (31) Number completed treatment in intervention (control): 30 (29) Number included in analysis from intervention (control): 30 (29) Comorbidity: not specified Sex: 52 female, 7 male Ethnicity: not specified Other sample characteristics: n/a |
|
| Interventions |
|
|
| Outcomes |
Used in study but not review:
|
|
| Identification |
Date of study: original paper published 2012 Sponsorship source: research was funded by the Spanish Ministry of Science and Technology (SEJ2009‐02440) and the Valencian Regional Government of Industry, University and Science (GV06/373) Setting: stomatology clinic at Stomatology Department Valencia University General Hospital Country: Spain Author name: Maite Ferrando Institution: University of Valencia and Valencia University General Hospital, Valencia, Spain Email: teresa.ferrando@uv.es Address: Department of Personality, Assessment, and Psychologic Treatments, University of Valencia |
|
| Notes | This study is also reported in Dura‐Ferrendis 2017, which adds interesting insights but no additional data. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer programme for random number generation |
| Allocation concealment (selection bias) | Low risk | Allocation according to previously defined criteria and random number allocation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible to blind participants in this kind of trial. Study personnel blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No intention‐to‐treat analysis. Data from 11 people who dropped out of the intervention group not included |
| Selective reporting (reporting bias) | Low risk | Table of results presents all outcomes |
| Other bias | Low risk | None noted |
Gatchel 2006.
| Study characteristics | ||
| Methods |
Study design: parallel‐group RCT Number of control groups: 1 Number of intervention groups: 1 |
|
| Participants |
Inclusion criteria: adults age 18 to 70 years, jaw or facial pain present for less than 6 months Exclusion criteria: comorbid pain‐exacerbating physical condition (such as cancer or fibromyalgia), history of jaw pain before the most recent episode Pretreatment: no significant demographic differences at baseline. Number eligible for study: 101 Number of participants: 101 Number randomly assigned to intervention (control): 56 (45) Number started treatment in intervention (control): 56 (45) Number completed treatment in intervention (control): 54 (45) Number included in analysis from intervention (control): 54 (45) Comorbidity: major comorbidities excluded Sex: female 80.5%, male 19.5% Ethnicity: white 78.5%, Hispanic 12%, African American 8%, Asian 4.5%, other 3% Other sample characteristics: marital status, employment status, health/dental insurance, education, income, referrer |
|
| Interventions |
|
|
| Outcomes |
Used in study but not review:
|
|
| Identification |
Date of study: paper published 2006 Sponsorship source: supported in part by National Institutes of Health grants 2R01 DE10713, 2R01 MH46452 and 1K05 MH071892 Setting: specialist TMD clinic, TMD clinical research programme Country: USA Author name: Robert J Gatchel Institution: University of Texas at Arlington Email: gatchel@uta.edu Address: Department of Psychology, College of Science, University of Texas at Arlington, 313 Life ScienceBuilding, 501 S. Nedderman Drive, Arlington, Texas 76019‐0528 |
|
| Notes | Early intervention group participants were more likely to also seek additional treatment from a range of other practitioners during the study period, which could account for some differences in outcome. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Details of randomisation method not given |
| Allocation concealment (selection bias) | High risk | Details not given |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible to blind participants to this kind of treatment. Blinding of study personnel not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Blinding of outcome assessors not discussed |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 98 of 101 participants completed 1‐year follow‐up |
| Selective reporting (reporting bias) | Low risk | Clearly defined outcome measures with good rationale described and thoroughly reported |
| Other bias | Unclear risk | Note although mean duration of TMD is 97 days, it was not possible to separate participants who had experienced symptoms for less than 3 months. Participants in the treatment group were more likely to also seek other forms of treatment, which could influence outcome |
Litt 2010.
| Study characteristics | ||
| Methods |
Study design: parallel‐group RCT Number of control groups: 1 Number of intervention groups: 1 |
|
| Participants |
Inclusion criteria: positive Axis I diagnosis on the RDC/TMD bilateral or unilateral pain in the area of the TMJ that had persisted and was noticeable on a daily basis for a period of at least 3 months Exclusion criteria: contraindication to TMD treatment, lack of fluency in English (as determined by inability to read and understand a statement of informed consent), previous surgery for treatment of TMD pain, history of rheumatoid disease, extensive anatomical destruction or deterioration of the TMJ, diagnosed as having pain of neuropathic or odontogenic origin, diagnosis of psychosis, current use of antidepressants or anxiolytics, taking opioid pain medication, pregnancy (due to possible adverse effects in pregnancy with the prescription of non‐steroidal anti‐inflammatory drugs) Pretreatment: not explicitly addressed Number eligible for study: 121 Number of participants: 101 Number randomly assigned to intervention (control): 52 (49) Number started treatment in intervention (control): 52 (49) Number completed treatment in intervention (control): 52 (49) Number included in analysis from intervention (control): 52 (49) Comorbidity: not discussed other than exclusions Sex: 85 female, 16 male Ethnicity: white (79%), black (9%), Hispanic (9%), other (3%) Other sample characteristics: 41% married/cohabiting, average of 14.7 yrs education |
|
| Interventions |
|
|
| Outcomes |
Used in study but not review:
|
|
| Identification |
Date of study: October 2003 to July 2007, published 2009 to 2010 Sponsorship source: "Support for this project was provided by grants R01‐DE14607from the National Institute on Dental and Craniofacial Research and by General Clinical Research Center grant M01‐RR06192 from the National Institutes of Health." Setting: dental hospital ‐ university school of dental medicine Country: USA Author name: Mark D Litt Institution: University of Conneticut Email: Litt@nso.uchc.edu Address: Division of Behavioral Sciences and Community Health – MC3910, University of Connecticut Health Center, Farmington, CT 06030, USA |
|
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Stratified randomisation (computerised urn method) generated by an independent study co‐ordinator |
| Allocation concealment (selection bias) | High risk | Not discussed |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible for participants to be blinded to treatment in this type of trial. Study personnel were not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcome assessors not blinded as participant self‐report |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "At posttreatment 88% of patients provided data, and 73% provided data at 52 weeks. Losses to follow‐up were equivalent across treatment conditions." "Analysis of main effects of treatment on each of the three major dependent variables was conducted using a mixed model regression procedure (Proc MIXED, SAS Institute [25]), and an intent‐to‐treat approach." |
| Selective reporting (reporting bias) | Low risk | Model for mediators and moderators is well described and tested. Plausible outcome data are presented. |
| Other bias | Low risk | None noted |
Lupton 1968.
| Study characteristics | ||
| Methods |
Study design: parallel‐group RCT Number of control groups: 1 Number of intervention groups: 2 |
|
| Participants |
Inclusion criteria: not reported Exclusion criteria: not reported Pretreatment: no Number eligible for study: not reported Number of participants: 60 Number randomly assigned to intervention (control): 20, 20 (20) Number started treatment in intervention (control): 20, 20 (20) Number completed treatment in intervention (control): not stated Number included in analysis from intervention (control): not stated Comorbidity: not reported Sex: 49 female, 11 male Ethnicity: white (52) Other sample characteristics: median level of education = 12th grade, married, median age = 30 years, dominant category of personality diagnosis |
|
| Interventions |
|
|
| Outcomes | Used in study but not review:
|
|
| Identification |
Date of study: not reported Sponsorship source: not reported Setting: TMJ Research Center of the College of Dentistry of the University of Illinois Country: USA Author name: Daniel E Lupton Institution: University of Ilinois Email: none Address: University of Ilinois |
|
| Notes | Data extraction not completed as outcomes relevant to the review are not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Method not reported |
| Allocation concealment (selection bias) | High risk | Not concealed |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Main outcome is the subjective opinion of the treating dentist. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No information about how many participants completed treatment or were included in the analysis. |
| Selective reporting (reporting bias) | Low risk | All outcomes measured appear to be reported. |
| Other bias | High risk | Main outcome measure is a subjective judgement by the treating dentist; author of the report also served as tutor/counsellor in both groups. |
Mishra 2000.
| Study characteristics | ||
| Methods |
Study design: parallel‐group RCT Number of control groups: 1 Number of intervention groups: 3 |
|
| Participants |
Inclusion criteria: participants must have endorsed past or present jaw or facial pain, clicking, popping, or locking of the jaw or have received a past diagnosis of TMD Exclusion criteria: significant physical health condition (e.g. cancer, multiple sclerosis, carpal tunnel syndrome, fibromyalgia); 6 or more DSM‐IV Axis I diagnoses, psychosis, or active suicidal ideation; score 15 or below on the CPI (considered “doing well” and not in need of treatment) Pretreatment: no significant group differences at intake Number eligible for study: not stated Number of participants: 94 Number randomly assigned to intervention (control): 22, 23, 24 (25) Number started treatment in intervention (control): not stated Number completed treatment in intervention (control): 22, 23, 24 (25) Number included in analysis from intervention (control): 22, 23, 24 (25) Comorbidity: people with significant comorbidities including cancer and other pain conditions excluded, otherwise not mentioned Sex: 77 female, 17 male Ethnicity: white 74, African‐American 8, Hispanic 10, other 2 Other sample characteristics: mean education 15.54 years |
|
| Interventions |
|
|
| Outcomes |
Used in study but not review:
|
|
| Identification |
Date of study: paper published 2000 Sponsorship source: grants ROI DE10713 and K02 MH01107 National Institutes of Health Setting: university clinic Country: USA Author name: Kiran Mishra Institution: University of Texas Email: robert.gatchel@email.swmed.ed Address: Department of Psychiatry, Division of Psychology, The University of Texas Southwestern Medical Center at Dallas, 5323 Harry Hines Boulevard, Dallas, Texas 75235‐9044. |
|
| Notes | Includes Mishra 2000, Gardea 2001 and Bernstein 2000. There are some discrepancies in methods reported between these papers. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Stratified randomisation (urn method) of randomisation |
| Allocation concealment (selection bias) | High risk | Allocation concealment not discussed |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible to blind participants in this kind of study. No mention of blinding of personnel |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Self‐report; participants as own outcome assessors. No mention of blinding of study assessors |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Number of participants who provided outcome data is not stated. Differences between papers of the same study in number of participants included, Mishra 2000 states 94, Gardea 2001 states 108, Bernstein 2000 states 121. |
| Selective reporting (reporting bias) | High risk | Measures described include pain, disability and mood, which are primary outcomes for this kind of study. Inconsistency in results, for example, CPI pre‐ and post‐treatment scores reported but POMS change scores for each scale reported. |
| Other bias | Low risk | None noted |
Mora 2013.
| Study characteristics | ||
| Methods |
Study design: parallel‐group RCT Date of study: August 2008 to April 2011 Setting of intervention: university ‐ Marburg Dental School Number of separate groups included in the study: 2 Number of control groups: 1 Number of intervention groups: 1 |
|
| Participants |
Inclusion criteria: painful axis 1 TMD diagnosis according to the RDC/TMD diagnostic criteria. Pain present for at least 3 months. Age between 18 and 70 Exclusion criteria: patient already has an occlusal appliance, needs further diagnostic investigation or dental or maxillofacial treatment as judged by a specialised dentist, has other major chronic pain conditions predominant in disability such as chronic low back pain or headache, has major medical or psychiatric conditions that would interfere with the ability to participate, has no pain Eligible for study: 70 Total participants: 58 Randomly assigned to intervention (control): 29 (29) Started treatment in intervention (control): 29 (27) Completed treatment in intervention (control): 27 (27) Included in analysis from intervention (control): 29 (27) Pretreatment: no significant group differences at intake Comorbidity: pain comorbidities excluded Sex: not reported Ethnicity: not reported Other: seeking treatment at a university dental clinic; TMD diagnosis based on RDC for TMD |
|
| Interventions |
Intervention characteristics
|
|
| Outcomes | Primary
Secondary
Follow‐up assessment took place 6 months after the treatment. Used in study but not review:
|
|
| Identification |
Sponsorship source: none stated Country: Germany Setting: specialist dental clinic at a university Author name: Meike C Shedden Mora Institution: Marburg University Email: m.shedden@staff.uni‐marburg.de Address: Department of Clinical Psychology and Psychotherapy, Philipps University of Marburg, Gutenbergstr. 18, 35032 Marburg, Germany |
|
| Notes | Emailed Cochrane Oral Health on 29 April 2020 to request a translation or help with data extraction as article is written in German. Data summary provided. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random assignment to conditions was generated by a researcher not involved in the study, with the use of randomisation software (GraphPad Software Inc. La Jolla, CA). |
| Allocation concealment (selection bias) | Low risk | Assignment was concealed in closed envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding not possible for participants. Personnel blinded as far as possible |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors were blind to treatment status. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat analysis |
| Selective reporting (reporting bias) | Low risk | Outcomes are reported as described in the methods. |
| Other bias | Low risk | None identified |
NCT00066937.
| Study characteristics | ||
| Methods |
Study design: parallel‐group RCT Number of control groups: 2 Number of intervention groups: 2 |
|
| Participants |
Included criteria: age 18 to 65 years, pain of at least 3 months duration due to TMD, pain due to TMD is primary if other pain conditions present Excluded criteria: continuous, chronic painful non‐reducing disc displacement of TMJ and patient cannot open mouth, unstable or acute severe pain from another pain condition, pregnancy, presence of a medical condition that contraindicates nortriptyline: angle‐closure glaucoma, symptomatic orthosis, electrocardiogram: first degree heart block or QTc > 450 msec, unstable angina or a history of a myocardial infarction within the past 3 months, current treatment with an antidepressant that cannot be withdrawn, current use of a medication that interacts with nortriptyline to raise blood levels, such as selective serotonin reuptake inhibitors (e.g. paroxetine), systemic antifungal agents (fluconazole), antiarrhythmics (e.g. quinidine), antipsychotics (e.g. haloperidol) and antibiotics (e.g. erythromycin), presence of dementia, psychosis or other disorder of cognition that impairs ability to participate in minimal contact intervention, BDI score ≥ 35 or BDI Item #9 (suicide item) is scored > 1, terminal illness with a life expectancy of less than 6 months, history of arthrotomy of TMJ, history of allergic reaction to nortriptyline or benztropine, history of a therapeutic trial with nortriptyline (dose ≥ 100 mg for at least 3 weeks) Pretreatment: unclear Number eligible for study: not stated Number of participants: 140 Number randomly assigned to intervention (control): nortriptyline plus CBT 41, nortriptyline plus disease management 37, benztropine (placebo) plus CBT 38, benztropine (placebo) plus disease management 24 Number started treatment in intervention (control): 41 (38) Number completed treatment in intervention (control): nortriptyline plus CBT 38, nortriptyline plus disease management 26, benztropine (placebo) plus CBT 33, benztropine (placebo) plus disease management 19 Number included in analysis from intervention (control): nortriptyline plus CBT 38, nortriptyline plus disease management 33, benztropine (placebo) plus CBT 26, benztropine (placebo) plus disease management 19 Comorbidity: not stated Sex: 105 female, 35 male Ethnicity: not stated Other sample characteristics: n/a |
|
| Interventions |
|
|
| Outcomes |
Measured at baseline, post‐treatment, 3 months, 6 months Used in study but not review:
|
|
| Identification |
Date of study: November 2002 to June 2008, data reported 2017 Sponsorship source: National Institute of Dental and Craniofacial research Country: USA Setting: university Author name: Jennifer A Haythornthwaite Institution: University of Maryland Email: not known Address: University of Maryland, Dental School, Baltimore, Maryland, United States, 21201 |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "factorial assignment" |
| Allocation concealment (selection bias) | Unclear risk | Registry does not provide sufficient detail for a judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | States fourfold blinding but not enough information. Blinding of clinician not possible |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | States outcome assessors blinded but not enough information ‐ self‐report |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Analyses are not on intention‐to treat basis, significant dropout |
| Selective reporting (reporting bias) | High risk | Measures seem unusual and inconsistent, e.g. average pain versus change in pain‐related interference. |
| Other bias | High risk | Registered trial that was not published and therefore has not been peer‐reviewed. Very long timescale. Study data appear to have been collected over a long period of time. |
Shevtsova 2020.
| Study characteristics | ||
| Methods |
Study design: RCT Number of control groups: 1 Number of intervention groups: 1 |
|
| Participants |
Inclusion criteria: people with myofascial pain syndrome who attended the pain clinic Exclusion criteria: not stated Pretreatment: not reported Number eligible for study: not reported Number of participants: 64 people who reported TMD Number randomly assigned to intervention (control): 32 Number started treatment in intervention (control): 32 Number completed treatment in intervention (control): 32 Number included in analysis from intervention (control): 32 Comorbidity: not reported Sex: not reported Ethnicity: not reported Other sample characteristics: not reported |
|
| Interventions |
|
|
| Outcomes |
|
|
| Identification |
Date of study: not reported Sponsorship source: not reported Setting: not reported Country: Russia Author name: G Shevtsova Institution: The State Education Institution of HIgher Professional Training, The First Sechenov Moscow State Medical University under Ministry of Health of the Russian Federation Email: not reported Address: 8‐2, Trubetskaya Street, Moscow, 119992 |
|
| Notes | Conference presentation: abstract only available Limited information provided |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sealed envelopes |
| Allocation concealment (selection bias) | Low risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Self‐report; not possible |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Not stated |
| Selective reporting (reporting bias) | Unclear risk | Not enough information |
| Other bias | Unclear risk | Not enough information |
Stam 1984.
| Study characteristics | ||
| Methods |
Study design: parallel‐group RCT Number of control groups: 1 Number of intervention groups: 2 |
|
| Participants |
Inclusion criteria: diagnosed as suffering from TMPDS on the basis of lack of changes or organic disease of either TMJ as determined by radiographs, lack of tenderness of the condyles on physical examination, and presence of at least one of the following symptoms: pain and tenderness of the muscles of mastication, sounds during condylar movements, mainly clicking, and limitations of mandibular movements Exclusion criteria: not reported Pretreatment: not discussed Number eligible for study: not reported Number of participants: 61 Number randomly assigned to intervention (control): not reported Number started treatment in intervention (control): not reported Number completed treatment in intervention (control): 12 hypnosis, 15 relaxation (10 waiting‐list control). Number included in analysis from intervention (control): 12, 15 (10) Comorbidity: not discussed Sex: 51 female, 10 male Ethnicity: not reported Other sample characteristics: participants ranged in age from 15 to 41 years. Mean duration of pain before treatment was 23 months (standard deviation = 26); median duration was 12 months. |
|
| Interventions |
|
|
| Outcomes |
|
|
| Identification |
Date of study: paper published 1984 Sponsorship source: not reported Country: Canada Setting: dental hospital clinic, Department of Oral Medicine Author name: Henderikus J Stam Institution: University of Western Ontario, London, Ontario, Canada Email: n/a Address: Department of Psychology, University of Calgary, Calgary, Alberta, Canada T2N 1N |
|
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "After their pretest session, patients were randomly assigned to one of three treatments with the exception that each group had approximately equivalent numbers of subjects with high, medium, and low susceptibility to hypnosis: hypnosis (n = 12), relaxation (n = 15), or waiting‐list control (WCL) (n = 14)." |
| Allocation concealment (selection bias) | High risk | Not discussed |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible to blind for this kind of study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Self‐report ratings cannot be blinded. Outcome assessor who gave judgement about improvement was blind to what treatment participants had received. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Does not state whether intention‐to‐treat analysis was carried out. All participants completed daily pain ratings but results of these were only presented for 10 waiting‐list control participants. At end of treatment only dentist judgements of improvement were presented. |
| Selective reporting (reporting bias) | High risk | Outcomes appear to have been decided once data collected. |
| Other bias | High risk | Subjective judgement of dentist regarding whether or not participants had improved. |
Townsend 2001.
| Study characteristics | ||
| Methods |
Study design: parallel‐group RCT Number of control groups: 1 Number of intervention groups: 1 |
|
| Participants |
Inclusion criteria: positive screen for TMD using self‐report format adapted from TMD criteria outlined by AAOP 1996 and Bush 1995. The criteria include a report of pain in the TMJ or surrounding musculature in the past year (a) locked jaw; (b) mandibular joint sounds; (c) stiffness, tenderness, or tightness in the jaw; (d) pain in the ears, temple or cheek; or (e) uncomfortable bite. Exclusion criteria: (a) having had head or facial surgery, (b) diagnosis of degenerative joint disorder, (c) currently taking psychotropic medication, or (d) pregnancy Pretreatment: at baseline, the control group reported higher educational level than the treatment group. No other significant differences Number eligible for study: 24 Number of participants: 20 Number randomly assigned to intervention (control): not stated Number started treatment in intervention (control): 10 (10) Number completed treatment in intervention (control): not exactly clear as article states 10 (10), but also mentions imputation of data using the last available score Number included in analysis from intervention (control): 10 (10) Comorbidity: not discussed Sex: all female Ethnicity: all white Other sample characteristics: 100% employed full time, none receiving non‐pharmacological treatment, 80% reported use of intra‐oral appliance |
|
| Interventions |
|
|
| Outcomes |
Used in study but not review:
|
|
| Identification |
Date of study: published 2001, recruitment over 15‐month period Sponsorship source: none ‐ student dissertation Country: USA Setting: not stated. Minimal contact so mainly in participants' homes, intake probably in university psychology department Author name: Donald Townsend Institution: Virginia Commonwealth University Email: corresponding author: sgramlin@mail1.vcu.edu Address: Metropolitan Sleep Disorders Center, St. Paul, MN, USA; Department of Psychology, Virginia Commonwealth University, Box 2018, Richmond, VA, USA |
|
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Blocked randomisation table with numbers to represent each treatment condition. Partially randomised as participants who dropped out prior to treatment were replaced by the next available person. The advanced student who also acted as therapist and researcher performed the randomisation. |
| Allocation concealment (selection bias) | High risk | Group therapist assigned numbers on the block randomisation to participants after conducting a pre‐treatment assessment. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible to blind participants to this kind of study. Study personnel aware of treatment allocation. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Self‐report; participants as own outcome assessors; no information about who processed the outcome data. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "the fact that information is only available for those who completed the study (thus not allowing for the preferred ‘‘intent‐to‐treat’’ analysis) should give pause to overgeneralization of the current findings." Comment: information is not presented clearly about treatment dropout. |
| Selective reporting (reporting bias) | Low risk | Primary outcomes of interest to the review are reported. |
| Other bias | Low risk | None noted |
Turk 1993.
| Study characteristics | ||
| Methods |
Study design: parallel‐group RCT Number of control groups: 1 Number of intervention groups: 2 |
|
| Participants |
Inclusion criteria: pain and tenderness of the muscles of mastication and TMJ region and limited mandibular movements of 2 months duration or longer, no evidence of serious psychopathology, no history of TMJ‐related surgery, and at least 18 years of age Exclusion criteria: not specified Pretreatment: none Number eligible for study: not stated Number of participants: 80 Number randomly assigned to intervention (control): 30, 30 (20) Number started treatment in intervention (control): not stated Number completed treatment in intervention (control): not stated Number included in analysis from intervention (control): not stated Comorbidity: not reported Sex: not reported Ethnicity: not reported Other sample characteristics: n/a |
|
| Interventions |
|
|
| Outcomes |
|
|
| Identification |
Date of study: paper published 1993 Sponsorship source: USPHS Research Grant ROl DE07514 from the NIDR, National Institutes of Health Country: USA Setting: university clinic Comments: data from study 2 (combined group) not included in data synthesis as this part of the trial was not randomised Author name: Dennis C Turk Institution: University of Pittsburgh Email: none provided Address: Department of Psychiatry; Director of Pain Evaluation and Treatment Institute, University of Pittsburgh School of Medicine |
|
| Notes | This paper reports on 2 studies; participants in study 1 were randomised, but in study 2 the next 30 consecutive patients referred for treatment composed the study group. Only study 1 is included in the review. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Sequence generation not discussed |
| Allocation concealment (selection bias) | High risk | Allocation concealment not discussed |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No discussion of blinding. Blinding is not possible for this type of intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Blinding of outcome assessors not discussed |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Treatment dropout is low, although no intention‐to‐treat analysis |
| Selective reporting (reporting bias) | Low risk | Data are reported for all measures described in the methods. |
| Other bias | Low risk | None noted |
Turk 1996.
| Study characteristics | ||
| Methods |
Study design: parallel‐group RCT Number of control groups: 1 Number of intervention groups: 1 |
|
| Participants |
Inclusion criteria: pain and tenderness of the muscles of mastication and TMI region and restricted mandibular opening of 3 months duration or longer, no evidence of serious psychopathology, no history of TMJ‐related surgeries and at least 18 years of age Exclusion criteria: not explicitly stated. None of the participants were receiving disability payments in relation to their pain. Pretreatment: no significant differences between groups at baseline. Number eligible for study: not stated Number of participants: 48 Number randomly assigned to intervention (control): 24 (24) Number started treatment in intervention (control): 24 (24) Number completed treatment in intervention (control): 22 (23) Number included in analysis from intervention (control): 21 (20) Comorbidity: not reported Sex: 90% female, 10% male Ethnicity: not reported Other sample characteristics: high school graduation 92%, single 25%, married 68%, separated/divorced 7%, full‐time employment 61% |
|
| Interventions |
|
|
| Outcomes |
Used in study but not review:
|
|
| Identification |
Date of study: published 1996 Sponsorship source: US Public Health Service Research Grant R01 DE07514 from the NIDR, National Institutes of Health Country: USA Setting: University of Pittsburgh Medical Centre Author name: Dennis C Turk Institution: University of Pittsburgh Email: not reported Address: Dennis C Turk, Pain Evaluation and Treatment Institute, University of Pittsburgh Medical Center, 4601 Banm Boulevard, Pittsburgh, Pennsylvania 15213 |
|
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Method of sequence generation not discussed |
| Allocation concealment (selection bias) | High risk | Allocation concealment not discussed. Participants were all allocated to an active treatment. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding not discussed. Blinding of participants and treatment providers not possible for this kind of study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Self‐report ‐ participants as own outcome assessors. Blinding of study assessors not discussed |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low dropout, although does not use intention‐to‐treat analyses. 22 and 23 out of 24 completed the treatment and 21 and 22 reached 3‐month follow‐up |
| Selective reporting (reporting bias) | Low risk | Outcomes appear to be comprehensively reported, including core outcomes of pain and interference. |
| Other bias | Low risk | None noted |
Turner 2006.
| Study characteristics | ||
| Methods |
Study design: parallel‐group RCT Number of control groups: 1 Number of intervention groups: 1 |
|
| Participants |
Inclusion criteria: age 18 years or older, RDC/TMD Axis I TMD diagnosis Dworkin 1992 made by an oral medicine specialist based on a structured RDC/TMD clinical examination, residence within a 2‐hour drive of the TMD clinic, facial pain for at least 3 months, facial pain‐related disability, as defined by a chronic pain grade of II high, III, or IV (Von Korff 1992) and ability to communicate in English Exclusion criteria: need for further diagnostic evaluation, pending litigation or disability compensation for pain, current or previous CBT for pain, and major medical or psychiatric conditions that would interfere with ability to participate Pretreatment: similar demographically at baseline. More self‐care patients had disc displacement and more participants in PMT group had osteoarthritis of the jaw joint. Number eligible for study: 366 Number of participants: 158 Number randomly assigned to intervention (control): 79 (79) Number started treatment in intervention (control): 77 (79) Number completed treatment in intervention (control): 57 (53) completed all sessions, 67 (69) completed some sessions Number included in analysis from intervention (control): 72 (76) Comorbidity: not discussed Sex: 86% female, 14% male Ethnicity: 84% white Other sample characteristics: referred to specialist clinic |
|
| Interventions |
|
|
| Outcomes |
Used in study but not review:
|
|
| Identification |
Date of study: 2001 to 2004, paper published 2006 Sponsorship source: funding for this study was provided by the National Institute of Dental and Craniofacial Research Grant P01 DE08773 Country: USA Setting: Specialist Orofacial Pain Clinic Author name: Judith A Turner Institution: University of Washington Email: jturner@u.washington.edu Address: Department of Psychiatry and Behavioral Sciences, University of Washington School of Medicine, Seattle, WA, USA |
|
| Notes | One of the three papers relating to this study was additional analyses of mediators and moderators, but had no additional raw data (Turner 2007). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Generated by a biostatistician on a computer programme using stratified sampling in random block designs |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible to blind participants due to nature of study. Randomsation assignment was concealed to all study personnel until envelopes were opened by research staff after participant consent was obtained. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Blinding of outcome assessors not mentioned. Self‐report so participants largely acting as their own outcome assessors and cannot be blind to the treatment they have received. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Clear rationale of imputation and sensitivity analyses where intention‐to‐treat analysis not possible due to dropout |
| Selective reporting (reporting bias) | Low risk | Predetermined endpoints for analysis. Table reports data for all key measures collected. |
| Other bias | Low risk | None noted |
Turner 2011.
| Study characteristics | ||
| Methods |
Study design: parallel‐group RCT How many control groups: 1 How many intervention groups: 2 |
|
| Participants |
Inclusion criteria: female, age 18 to 45 years, premenopausal, RDC/TMD Axis I TMD pain diagnosis made by an oral medicine specialist based on a structured RDC/TMD clinical examination, characteristic pain intensity ≥ 3 (0 to 10 scale, past 6 months timeframe), ability to communicate in English Exclusion criteria: lacking menstrual cycle, pregnant, lactating, planning to become pregnant in next 7 months, unwilling to take a continuous oral contraceptive, need for further diagnostic evaluation of facial pain (determined by oral medicine specialist), major medical/psychiatric comorbidities that would interfere with ability to participate, medical contraindication for continuous oral contraceptive therapy, smoked and ≥ 35 years old, used medication in last 3 months that interfered with oestrogen or progestin metabolism, abnormal pelvic exam abnormal pap smear, undiagnosed uterine bleeding, no current mammogram and ≥ 40 years old Pretreatment: targeted self‐management therapy group significantly younger, although all groups' average age 25 to 29. No other significant differences between groups. Number eligible for study: 570 Number of participants: 191 Number randomly assigned to intervention (control): 60 (57) (74) Number started treatment in intervention (control): (55) (52) (36) Number completed treatment in intervention (control): self‐management therapy 54 (completed at least 1 session), targeted self‐management therapy 50, continuous oral contraceptive therapy 36 Number included in analysis from intervention (control): (51) (47) (49) Comorbidity: none specified Sex: all female Ethnicity: 78% white non‐Hispanic Other sample characteristics: menstruating |
|
| Interventions |
|
|
| Outcomes |
Used in study but not review:
|
|
| Identification |
Date of study: recruitment occurred between 2005 and 2009, paper published 2011 Sponsorship source: National Institute of Dental and Craniofacial Research Country: USA Setting: university orofacial pain clinic Author name: Judith A Turner Institution: University of Washington Email: jturner@u.washington.edu Address: University of Washington School of Medicine, Department of Psychiatry and Behavioral Sciences, Box 356560, Seattle, WA 98195, USA |
|
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Stratified block randomisation undertaken by study assistant not involved in screening using a computer programme |
| Allocation concealment (selection bias) | Low risk | Sequence put into opaque, sequentially numbered envelopes by an assistant not otherwise involved in the study |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible to blind participants to the intervention received in this type of trial. Study assistant was blinded. All study personnel remained blind to assignment until the point of randomisation. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not possible to blind for self‐report outcome questionnaires. Blinding of outcome assessors is not explicitly reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing data clearly described and sensitivity analysis carried out regarding imputation and intention‐to‐treat strategy. |
| Selective reporting (reporting bias) | Unclear risk | Pain is primary outcome; pain interference and BDI also core outcomes for this population so good coverage; however, many questionnaires used and not all are reported in detail |
| Other bias | Low risk | None noted |
Wahlund 2003.
| Study characteristics | ||
| Methods |
Study design: parallel‐group RCT How many control groups: 2 (occlusal appliance designated by review author as control group) How many intervention groups: 1 |
|
| Participants |
Inclusion criteria: TMD diagnosis according to RDC/TMD, reported pain once a week or more for 3 months in jaw, face or temples. Age 12 to 18 years Exclusion criteria: juvenile rheumatoid arthritis, migraine, current treatment with orthodontic appliances that could interfere with treatment Pretreatment: groups seem to be relatively similar; more females than males, which reflects the population with TMD Number eligible for study: not stated Number of participants: 122 Number randomly assigned to intervention (control): relaxation 41, oral appliance 42 (39) Number started treatment in intervention (control): 41, 42 (39) Number completed treatment in intervention (control): 34, 37 (39) Number included in analysis from intervention (control): 34, 37 (39) Comorbidity: see exclusion criteria above Sex: 93 female, 29 male Ethnicity: not reported Other sample characteristics: n/a |
|
| Interventions |
|
|
| Outcomes |
Used in study but not review:
|
|
| Identification |
Date of study: 2003. Data from 1996 to 2000 Sponsorship source: Public Dental Service of Ostergotland Country: Sweden Setting: specialist TMD clinic Author name: Kerstin Wahlund Institution: TMD Unit, Specialist Center for Oral Rehabilitation, LinkoÈping, Sweden Email: kerstin.Wahlund@lio.se Address: TMD Unit, Specialist Center for Oral Rehabilitation, Torkelbergsgatan 11, SE‐581 85 LinkoÈping, Sweden. Tel. +46 13 228850, fax. +46 13 228847. |
|
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | No mention of randomisation process |
| Allocation concealment (selection bias) | High risk | No mention of allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and personnel not blinded but unable to do this in this study design |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Examiner was blind to treatment allocation. Self‐report measures. At each evaluation all subjects filled out a self‐administered questionnaire and were clinically examined by a 'blinded' calibrated clinician. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 12% dropout, which was comparable between groups. Analysis was on the basis of treatment completers. Despite reporting of dropouts, appears > 10% in both intervention groups dropped out and were not included in analysis. |
| Selective reporting (reporting bias) | Low risk | All outcomes described in the methods are reported. |
| Other bias | Low risk | None noted |
Wahlund 2015.
| Study characteristics | ||
| Methods | Study design: parallel‐group RCT (cross‐over study considered as parallel group as only first period data used) | |
| Participants |
Inclusion criteria: age 12 to 19 years, TMD pain at least once a week for 3 months, RDC/TMD diagnosis of myofascial pain, seeking treatment Exclusion criteria: myofascial diagnosis or other diagnosis such as juvenile idiopathic arthritis or migraine, receiving orthodontic treatment that might interfere with treatment Pretreatment: no significant differences at baseline Number eligible for study: 111 Number of participants: 64 Number randomly assigned to intervention (control): 31 (33) Number started treatment in intervention (control): 30 (29) Number completed treatment in intervention (control): 28 (29) Number included in analysis from intervention (control): 28 (29) Comorbidity: not stated Sex: 61 female, 3 male Ethnicity: not reported Other sample characteristics: n/a |
|
| Interventions |
|
|
| Outcomes |
Used in study but not review:
|
|
| Identification |
Date of study: published 2015. Study conducted between September 2003 and January 2011 Sponsorship source: The Swedish Dental Society, The Public Dental Service of Ostergotland, Public Dental Service of Kalmar Country: Sweden Setting: specialist dental settings for TMD pain Author name: Kerstin Wahlund Institution: TMD Unit, Specialist Center for Oral Rehabilitation, LinkoÈping, Sweden Email: Kerstin.Wahlund@ltkalmar.se Address: Senior Consultant, Department of Stomatognathic Physiology, Kalmar County Hospital, Kalmar, Sweden |
|
| Notes | Pain index only partially reported This is reported as a cross‐over study, but we have only considered the first part of the study, which was designed as a standard RCT comparing relaxation and occlusal appliance. Longest follow‐up was 6 months and included only people who were satisfied with current pain and therefore did not wish to go into the cross‐over trial of the occlusal appliance. Data not suitable for combining in a meta‐analysis |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sequences generated from a random number table and put in opaque envelopes Quote: "random number table, a secretary not otherwise involved in the study generated the allocation sequence to assign patients to a treatment, either OA or RT." |
| Allocation concealment (selection bias) | Low risk | Secretary otherwise not involved in the study used the random number table and put assigned numbers into opaque envelopes. Envelopes were opened by a trained nurse after gathering patient information. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible for participants, healthcare workers providing interventions, and other personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors blinded. Quote: "Two previously calibrated TMD specialists (KW and IMN), blinded to group assignment, performed the examination." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dropout rates similar between groups: 3 (9.7%) in relaxation therapy and 4 (12.1%) with oral appliance. Dropouts excluded from data analysis but we found similar results with intention‐to‐treat analysis using imputations. |
| Selective reporting (reporting bias) | Low risk | All outcomes described in the methods are reported in the results. |
| Other bias | Low risk | None noted |
BDI: Beck Depression Inventory; CBT: cognitive behaviour therapy; CES‐D: Centre for Epidemiological Studies in Depression; DSM‐IV: Diagnostic and Statistical Manual of Mental Disorders, fourth edition; GAD‐7: General Anxiety Disorder 7‐item questionnaire; GCPS: Graded Chronic Pain Scale; MMPI: Minnesota Multiphasic Personality Inventory; MPI: Multidimensional Pain Inventory; n/a: not applicable; NIDR: National Institute of Dental Research; NRS: numerical rating scale; OA: occlusal appliance; PMT: pain management training; POMS: Profile of Mood States; RCT: randomised controlled trial; RDC: research diagnostic criteria; RT: relaxation therapy; SCL: symptom checklist; TMD: temporomandibular disorder; TMJ: temporomandibular joint; TMPDS: temporomandibular pain and dysfunction syndrome; VAS: visual analogue scale; USPHS: United States Public Health Service; WHYMPI: West Haven‐Yale Multidimensional Pain Inventory
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Carlson 2001 | Does not fit our criteria for psychological intervention. |
| Conti 2015 | Does not fit our criteria for psychological intervention. |
| Crockett 1986 | Does not fit our criteria for psychological intervention. |
| de Resende 2019 | Does not fit our criteria for psychological intervention. |
| Ferrando 2012b | Full text not available |
| Flor 1993 | Data were not presented separately for participants with TMD. |
| Funch 1984 | Does not fit our criteria for psychological intervention. |
| Halmova 2017 | Does not fit our criteria for psychological intervention. |
| Huhtela 2019 | Does not fit our criteria for psychological intervention. |
| Lam 2020 | Does not fit our criteria for psychological intervention. |
| Litt 2013 | Conference proceedings ‐ not enough information |
| Manfredini 2017 | Does not fit our criteria for psychological intervention. |
| Massouth 1992 | Conference proceedings ‐ not enough information |
| Melo 2020 | Does not fit our criteria for psychological intervention. |
| Michelotti 2002 | Does not fit our criteria for psychological intervention. |
| Ommerborn 2007 | Participants not diagnosed with TMD. |
| Pfeil 1987 | Full text not available |
| Takeuchi Sato 2019 | Does not fit our criteria for psychological intervention. |
| Treacy 1999 | Participants not diagnosed with TMD. |
| van Grootel 2017 | Does not fit our criteria for psychological intervention. |
TMD: temporomandibular disorder
Characteristics of ongoing studies [ordered by study ID]
CTRI2007091000047.
| Study name | A clinical trial to study the effects of three physiotherapy treatments ‐ jaw muscle exercise, myofascial release and cognitive behavioral therapy in temporomandibular muscle pain patients |
| Methods | Multi‐arm parallel RCT Random sequence generation: random number table. Method of allocation concealment: sequentially numbered, sealed, opaque envelopes Blinding: outcome assessor blinded |
| Participants | TMJ myofascial pain syndrome: localised pain on palpation of jaw muscles, diffuse pain reproduced to head or face, pain on resisted contraction of the jaw, passive range of jaw opening is greater than active pain‐free range |
| Interventions |
|
| Outcomes |
All measured day 1 before treatment and day 5 after 5 treatment sessions |
| Starting date | 1 Febuary 2008 |
| Contact information | Senthil Kumar Paramasivam, mptmanip@yahoo.com Manipal University, India |
| Notes |
JPRN‐C000000365.
| Study name | Randomized controlled clinical trial of cognitive behavioral therapy for chronic muscle pain in head, neck and shoulder regions |
| Methods | Parallel RCT |
| Participants | Target: 166 Age 16 to 55 years Inclusion criteria: age 16 to 55 years, chronic pain in masticatory muscles more than 4 weeks, pain ranked more than 4 on a VAS over 4 weeks until starting trial, able to go to hospital during the 26 weeks of the study Exclusion criteria: undergone treatment for TMD, pain caused by abnormal shape of bone, adhesion, disk displacement/perforation in TMJ, unable to fit splint to jaw because of missing teeth, judged by dentists not to be appropriate for this trial |
| Interventions |
|
| Outcomes |
|
| Starting date | 1 March 2006 |
| Contact information | Shoichi Ishigaki, ishigaki‐s@umin.net |
| Notes | Sponsor: Osaka University Graduate School of Dentistry Funder: Japan Society for the Promotion of Science |
NCT00067366.
| Study name | Brief treatment for temporomandibular pain |
| Methods | Open‐label parallel RCT |
| Participants |
Inclusion criteria
Exclusion criteria
|
| Interventions |
6 clinic visits in each intervention |
| Outcomes |
|
| Starting date | October 2003 |
| Contact information | Mark Litt, Professor, University of Connecticut Health Center, USA |
| Notes |
NCT00769561.
| Study name | Biofeedback‐based cognitive behavioral treatment for temporomandibular disorders |
| Methods | Single‐blind parallel RCT |
| Participants |
Inclusion criteria
Exclusion criteria
|
| Interventions |
|
| Outcomes |
Primary outcome measures
Secondary outcome measures
All outcomes measured pre‐treatment, post‐treatment after 8 weeks, and at 6‐month follow‐up |
| Starting date | August 2008 |
| Contact information | Winfried Rief, Philipps University Marburg Medical Center |
| Notes |
NCT04363762.
| Study name | An internet‐based multimodal pain program for chronic temporomandibular disorder pain |
| Methods | Unblinded parallel‐arm RCT pilot study |
| Participants |
Inclusion criteria
Exclusion criteria
|
| Interventions | Internet‐based multimodal pain programme versus occlusal splint |
| Outcomes | Primary
Secondary
All measured at 3 and 6 months of follow‐up |
| Starting date | April 2016 |
| Contact information | Professor Per Alstergren, Malmö University |
| Notes |
CBT: cognitive behavioural therapy; CES‐D: Center for Epidemiologic Studies Depression Scale; DC/TMD: Diagnostic Criteria for Temporomandibular Disorders; MRI: magnetic resonance imaging; NRS: numeric rating scale; RCT: randomised controlled trial; TM: temporomandibular; TMJ: temporomandibular joint; TMD: temporomandibular disorder; VAS: visual analogue scale
Differences between protocol and review
According to the protocol, we had planned to consider separate analyses of cognitive behaviour therapy (CBT), behaviour therapy (BT) and other therapies. We did not do this because only a very small number of studies reported interventions other than CBT. Of these studies, we judged that the high clinical heterogeneity would make it meaningless to consider them in the groupings specified in the original protocol. Instead, we reported all psychological therapies combined and a subset of CBT.
We had intended to include all outcomes in our summary of findings tables, but instead we focused on our primary outcomes and one secondary outcome, 'psychological distress'.
Contributions of authors
Chris Penlington designed the review, screened abstracts and full‐text studies, carried out risk of bias assessments, extracted data, carried out GRADE evidence certainty assessments and drafted and revised the full report. Charlotte Bowes and Greig Taylor, screened abstracts and full‐text studies, carried out risk of bias assessments, extracted data and contributed to the full report. Adetunji Adebowale Otemade, screened abstracts and drafted and revised the full report. Paula Waterhouse screened abstracts and drafted and revised the full report. Justin Durham carried out GRADE evidence certainty assessments, commented on and revised the full report. Richard Ohrbach designed the review, screened full‐text studies and commented on and revised the full report.
Sources of support
Internal sources
-
Manchester Academic Health Sciences Centre (MAHSC) and the National Institute for Health Research (NIHR) Manchester Biomedical Research Centre, UK
Support to Cochrane Oral Health
-
Division of Dentistry, School of Medical Sciences, Faculty of Biology, Medicine and Health, The University of Manchester, UK
Support to Cochrane Oral Health
External sources
-
National Institute for Health Research (NIHR), UK
This project was supported by the NIHR, via Cochrane Infrastructure funding to Cochrane Oral Health. The views and opinions expressed herein are those of the review authors and do not necessarily reflect those of the Evidence Synthesis Programme, the NIHR, the NHS, or the Department of Health and Social Care
-
Cochrane Oral Health Global Alliance, Other
The production of Cochrane Oral Health reviews has been supported financially by our Global Alliance since 2011 (oralhealth.cochrane.org/partnerships-alliances). Contributors in recent years have been the American Association of Public Health Dentistry, USA; AS‐Akademie, Germany; the British Association for the Study of Community Dentistry, UK; the British Society of Paediatric Dentistry, UK; the Canadian Dental Hygienists Association, Canada; the Centre for Dental Education and Research at All India Institute of Medical Sciences, India; the National Center for Dental Hygiene Research & Practice, USA; New York University College of Dentistry, USA; and Swiss Society of Endodontology, Switzerland
Declarations of interest
Chris Penlington: no conflict of interest Charlotte Bowes: no conflict of interest Greig Taylor: is funded by a National Institute for Health Research (NIHR) Doctoral Research Fellowship. The views expressed are his own and not necessarily those of the NHS, the NIHR, or the Department of Health and Social Care. Adetunji Adebowale Otemade: no conflict of interest Paula Waterhouse: no conflict of interest Justin Durham: no conflict of interest Richard Ohrbach: is a paid statistical consultant for the Journal of Prosthetic Dentistry and an Associate Editor for the Journal of Oral & Facial Pain and Headache, and he is funded by his university for research‐related travel. He receives honoraria and travel reimbursement for speaking engagements based on his published research. Private investments are unrelated to his scholarly activities.
New
References
References to studies included in this review
Abrahamsen 2011 {published data only}
- Abrahamsen R, Baad-Hansen L, Zachariae R, Svensson P. Effect of hypnosis on pain and blink reflexes in patients with painful temporomandibular disorders. Clinical Journal of Pain 2011;27(4):344-51. [DOI: 10.1097/AJP.0b013e3181ffbfcb] [DOI] [PubMed] [Google Scholar]
- Abrahamsen R, Zachariae R, Svensson P. Effect of hypnosis on oral function and psychological factors in temporomandibular disorders patients. Journal of Oral Rehabilitation 2009;36(8):556-70. [DOI] [PubMed] [Google Scholar]
- Nilsson 2007. Incidence and temporal patterns of temporomandibular disorder among Swedish adolescents. Journal of Orofacial Pain 2007;21(2):127-32. [PubMed] [Google Scholar]
Bartley 2019 {published data only}
- Bartley EJ, LaGattuta NR, Robinson ME, Fillingim RB. Optimizing resilience in orofacial pain: a randomized controlled pilot study on hope. Pain Report 2019;4(2):e726. [DOI] [PMC free article] [PubMed] [Google Scholar]
Calderon 2011 {published data only}
- Calderon PS, Tabaquim ML, Oliveira LC, Camargo AP, Netto TC, Conti PC. Effectiveness of cognitive-behavioral therapy and amitriptyline in patients with chronic temporomandibular disorders-a pilot study. Brazilian Dental Journal 2011;22(5):415-21. [DOI: 10.1590/S0103-64402011000500012] [DOI] [PubMed] [Google Scholar]
Dworkin 1994 {published data only}
- Dworkin SF, Turner JA, Wilson L, Massoth D, Whitney C, Huggins KH, et al. Brief group cognitive-behavioral intervention for temporomandibular disorders. Pain 1994;59(2):175-87. [DOI: 10.1016/0304-3959(94)90070-1] [DOI] [PubMed] [Google Scholar]
Dworkin 2002a {published data only}
- Dworkin SF, Huggins KH, Wilson L, Mancl L, Turner J, Massoth D, et al. A randomized clinical trial using research diagnostic criteria for temporomandibular disorders-axis II to target clinic cases for a tailored self-care TMD treatment program. Journal of Orofacial Pain 2002;16(1):48-63. [PubMed] [Google Scholar]
Dworkin 2002b {published data only}
- Dworkin SF, Turner JA, Mancl L, Wilson L, Massoth D, Huggins KH, et al. A randomized clinical trial of a tailored comprehensive care treatment program for temporomandibular disorders. Journal of Orofacial Pain 2002;16(4):259-76. [PubMed] [Google Scholar]
Ferrando 2012 {published data only}
- Dura-Ferrandis E, Ferrando-Garcia M, Galdon-Garrido MJ, Andreu-Vaillo Y. Confirming the mechanisms behind cognitive-behavioural therapy effectiveness in chronic pain using structural equation modeling in a sample of patients with temporomandibular disorders. Clinical Psychology and Psychotherapy 2017;24(6):1377-83. [DOI] [PubMed] [Google Scholar]
- Ferrando M, Galdon MJ, Dura E, Andreu Y, Jimenez Y, Poveda, R. Enhancing the efficacy of treatment for temporomandibular patients with muscular diagnosis through cognitive-behavioral intervention, including hypnosis: a randomized study. Oral Surgery, Oral Medicine, Oral Pathology and Oral Radiology 2012;113(1):81-9. [DOI: 10.1016/j.tripleo.2011.08.020] [DOI] [PubMed] [Google Scholar]
Gatchel 2006 {published data only}
- Gatchel RJ, Stowell AW, Wildenstein L, Riggs R, Ellis E 3rd. Efficacy of an early intervention for patients with acute temporomandibular disorder-related pain: a one-year outcome study. Journal of the American Dental Association 2006;137(3):339-47. [DOI: 10.14219/jada.archive.2006.0183] [DOI] [PubMed] [Google Scholar]
- Stowell AW, Gatchel RJ, Wildenstein L. Cost-effectiveness of treatments for temporomandibular disorders: biopsychosocial intervention versus treatment as usual. Journal of the American Dental Association 2007;138(2):202-8. [DOI] [PubMed] [Google Scholar]
Litt 2010 {published and unpublished data}
- Litt MD, Porto PB. Determinants of pain treatment response and nonresponse: identification of TMD patient subgroups. Journal of Pain 2013;14(11):1502-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litt MD, Shafer DM, Ibanez CR, Kreutzer DL, Tawfik-Yonkers Z. Momentary pain and coping in temporomandibular disorder pain: exploring mechanisms of cognitive behavioural treatment for chronic pain. Pain 2009;145(1-2):160-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litt MD, Shafer DM, Kreutzer DL. Brief cognitive-behavioral treatment for TMD pain: long-term outcomes and moderators of treatment. Pain 2010;151(1):110-6. [DOI: 10.1016/j.pain.2010.06.030] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lupton 1968 {published data only}
- Lupton D. In: Differential patient response to instruction, counseling, and dental treatment. National Seminar on Adult Education Research; 1968 Feb 11-13; Chicago. Chicago (IL): Chicago University, 1968.
Mishra 2000 {published data only}
- Bernstein DN, Gatchel RJ. Biobehavioral predictor variables of treatment outcome in patients with temporomandibular disorders 1. Journal of Applied Biobehavioral Research 2000;5(2):101-13. [Google Scholar]
- Gardea MA, Gatchel RJ, Mishra KD. Long-term efficacy of biobehavioral treatment of temporomandibular disorders. Journal of Behavioral Medicine 2001;24(4):341-59. [DOI] [PubMed] [Google Scholar]
- Mishra KD, Gatchel RJ, Gardea MA. The relative efficacy of three cognitive-behavioral treatment approaches to temporomandibular disorders. Journal of Behavioral Medicine 2000;23(3):293-309. [DOI: 10.1023/a:1005562126071] [DOI] [PubMed] [Google Scholar]
Mora 2013 {published data only}
- Mora MC, Bleichhardt G, Weber D, Neff A, Rief W. Biofeedback-based cognitive-behavioral treatment for chronic temporomandibular disorders: preliminary data from a randomized controlled trial [Biofeedback bei kraniomandibulären dysfunktionen: Vorläufige wirksamkeit und akzeptanz eines biofeedback-gestützten kognitiv-verhaltenstherapeutischen therapiekonzepts]. Psychotherapeut 2010;55(3):217-24. [Google Scholar]
- Mora MC, Weber D, Neff A, Rief W. Biofeedback-based cognitive-behavioral treatment compared with occlusal splint for temporomandibular disorder: a randomized controlled trial. Clinical Journal of Pain 2013;29(12):1057-65. [DOI: 10.1097/AJP.0b013e3182850559] [DOI] [PubMed] [Google Scholar]
NCT00066937 {published data only}
- NCT00066937. Comparison of psychological and pharmacological treatments for pain due to temporomandibular joint disorder (TMD). clinicaltrials.gov/show/nct00066937 (first received 8 August 2003).
Shevtsova 2020 {published data only}
- Shevtsova G, Medvedeva L, Zagorulko O, Vashchenko N. Sleep disorder symptoms improvement after the 12-week mindfulness therapy sessions in myofascial facial pain syndrome patients. European Journal of Neurology 2020;27 (Suppl 1):1227. [Google Scholar]
Stam 1984 {published data only}
- Stam HJ, McGrath PA, Brooke RI. The effects of a cognitive-behavioral treatment program on temporo-mandibular pain and dysfunction syndrome. Psychosomatic Medicine 1984;46(6):534-45. [DOI: 10.1097/00006842-198411000-00006] [DOI] [PubMed] [Google Scholar]
Townsend 2001 {published data only}
- Townsend D, Nicholson RA, Buenaver L, Bush F, Gramling S. Use of a habit reversal treatment for temporomandibular pain in a minimal therapist contact format. Journal of Behavior Therapy and Experimental Psychiatry 2001;32(4):221-39. [DOI: 10.1016/s0005-7916(02)00004-6] [DOI] [PubMed] [Google Scholar]
Turk 1993 {published data only}
- Turk DC, Zaki HS, Rudy TE. Effects of intraoral appliance and biofeedback/stress management alone and in combination in treating pain and depression in patients with temporomandibular disorders. Journal of Prosthetic Dentistry 1993;70(2):158-64. [DOI: 10.1016/0022-3913(93)90012-d] [DOI] [PubMed] [Google Scholar]
Turk 1996 {published data only}
- Turk DC, Rudy TE, Kubinski JA, Zaki HS, Greco CM. Dysfunctional patients with temporomandibular disorders: evaluating the efficacy of a tailored treatment protocol. Journal of Consulting and Clinical Psychology 1996;64(1):139-46. [DOI: 10.1037//0022-006x.64.1.139] [DOI] [PubMed] [Google Scholar]
Turner 2006 {published data only}
- Turner JA, Holtzman S, Mancl L. Mediators, moderators, and predictors of therapeutic change in cognitive-behavioral therapy for chronic pain. Pain 2007;127(3):276-86. [DOI: 10.1016/j.pain.2006.09.005] [DOI] [PubMed] [Google Scholar]
- Turner JA, Mancl L, Aaron LA. Brief cognitive-behavioral therapy for temporomandibular disorder pain: effects on daily electronic outcome and process measures. Pain 2005;117(3):377-87. [DOI: 10.1016/j.pain.2005.06.025] [DOI] [PubMed] [Google Scholar]
- Turner JA, Mancl L, Aaron LA. Short- and long-term efficacy of brief cognitive-behavioral therapy for patients with chronic temporomandibular disorder pain: a randomized, controlled trial. Pain 2006;121(3):181-94. [DOI: 10.1016/j.pain.2005.11.017] [DOI] [PubMed] [Google Scholar]
Turner 2011 {published data only}
- Turner JA, Mancl L, Huggins KH, Sherman JJ, Lentz G, LeResche L. Targeting temporomandibular disorder pain treatment to hormonal fluctuations: a randomized clinical trial. Pain 2011;152(9):2074-84. [DOI: 10.1016/j.pain.2011.05.005] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wahlund 2003 {published data only}
- Wahlund K, List T, Larsson B. Treatment of temporomandibular disorders among adolescents: a comparison between occlusal appliance, relaxation training, and brief information. Acta Odontologica Scandinavica 2003;61(4):203-11. [DOI: 10.1080/00016350310003891] [DOI] [PubMed] [Google Scholar]
Wahlund 2015 {published data only}
- Wahlund K, Nilsson IM, Larsson B. Treating temporomandibular disorders in adolescents: a randomized, controlled, sequential comparison of relaxation training and occlusal appliance therapy. Journal of Oral & Facial Pain and Headache 2015;29(1):41-50. [DOI: 10.11607/ofph.1285] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Carlson 2001 {published data only}
- Carlson CR, Bertrand PM, Ehrlich AD, Maxwell AW, Burton RG. Physical self-regulation training for the management of temporomandibular disorders. Journal of Orofacial Pain 2001;15(1):47-55. [PubMed] [Google Scholar]
Conti 2015 {published data only}
- Conti PC, Correa AS, Lauris JR, Stuginski-Barbosa J. Management of painful temporomandibular joint clicking with different intraoral devices and counseling: a controlled study. Journal of Applied Oral Science: Revista FOB 2015;23(5):529-35. [DOI: 10.1590/1678-775720140438] [DOI] [PMC free article] [PubMed] [Google Scholar]
Crockett 1986 {published data only}
- Crockett DJ, Foreman ME, Alden L, Blasberg B. A comparison of treatment modes in the management of myofascial pain dysfunction syndrome. Biofeedback and Self-regulation 1986;11(4):279-91. [DOI: 10.1007/bf01000164] [DOI] [PubMed] [Google Scholar]
de Resende 2019 {published data only}
- Resende CM, Oliveira Medeiros FG, Figueiredo Rego CR, Bispo AS, Barbosa GA, Almeida EO. Short-term effectiveness of conservative therapies in pain, quality of life, and sleep in patients with temporomandibular disorders: a randomized clinical trial. Cranio: the Journal of Craniomandibular Practice 2019;15:1-9. [DOI] [PubMed] [Google Scholar]
Ferrando 2012b {published data only}
- Ferrando M. A cognitive behavioral hypnosis protocol with demonstrated efficacy as part of the standard conservative treatment for temporomandibular patients: a randomized research with muscular diagnosis patients. Pain Medicine 2012;13(2):313-4. [Google Scholar]
Flor 1993 {published data only}
- Flor H, Birbaumer N. Comparison of the efficacy of electromyographic biofeedback, cognitive-behavioral therapy, and conservative medical interventions in the treatment of chronic musculoskeletal pain. Journal of Consulting and Clinical Psychology 1993;61(4):653-8. [DOI: 10.1037//0022-006x.61.4.653] [DOI] [PubMed] [Google Scholar]
Funch 1984 {published data only}
- Funch DP, Gale EN. Biofeedback and relaxation therapy for chronic temporomandibular joint pain: predicting successful outcomes. Journal of Consulting and Clinical Psychology 1984;52(6):928-35. [DOI: 10.1037//0022-006x.52.6.928] [DOI] [PubMed] [Google Scholar]
Halmova 2017 {published data only}
- Halmova K, Holly D, Stanko P. The influence of cranio-cervical rehabilitation in patients with myofascial temporomandibular pain disorders. Bratislavske Lekarske Listy 2017;118(11):710-3. [DOI] [PubMed] [Google Scholar]
Huhtela 2019 {published data only}
- Huhtela OS, Koivisto N, Hagg V, Sipila K. Effectiveness of applied relaxation method vs. splint in treatment of temporomandibular disorders in Finnish students. Journal of Oral Rehabilitation 2019;47(2):123-31. [DOI: 10.1111/joor.12884] [DOI] [PubMed] [Google Scholar]
Lam 2020 {published data only}
- Lam J, Svensson P, Alstergren P. Internet-based multimodal pain program with telephone support for adults with chronic temporomandibular disorder pain: randomized controlled pilot trial. Journal of Medical Internet Research 2020;22(10):e22326. [DOI] [PMC free article] [PubMed] [Google Scholar]
Litt 2013 {published data only}
- Litt M. The role of inflammation in cognitive-behavioral treatment for TMD pain. Journal of Pain 2013;14(Suppl 4):S93. [Google Scholar]
Manfredini 2017 {published data only}
- Manfredini D, Favero L, Cocilovo F, Monici M, Guarda-Nardini L. A comparison trial between three treatment modalities for the management of myofascial pain of jaw muscles: a preliminary study. Cranio 2017;36(5):327-31. [DOI: 10.1080/08869634.2017.1349571] [DOI] [PubMed] [Google Scholar]
Massouth 1992 {published data only}
- Massouth D, Wilson L, Dworkin S, Whitney C, Turner J, Huggins K, et al. Early cognitive-behavioral treatment for TMD-preliminary findings. Journal of Dental Research 1992;71(Spec Iss):185, Abstract no: 638. [Google Scholar]
Melo 2020 {published data only}
- Melo RA, Resende CM, Rego CR, Bispo AS, Barbosa GA, Almeida EO. Conservative therapies to treat pain and anxiety associated with temporomandibular disorders: a randomized clinical trial. International Dental Journal 2020;70(4):245-53. [DOI: 10.1111/idj.12546] [DOI] [PMC free article] [PubMed] [Google Scholar]
Michelotti 2002 {published data only}
- Michelotti A, Steenks MH, Farella M, Parisini F. Short-term effects of physiotherapy versus counselling for the treatment of myofascial pain of the jaw muscles. Journal of Oral Rehabilitation 2002;29(9):874. [Google Scholar]
Ommerborn 2007 {published data only}
- Ommerborn MA, Schneider C, Giraki M, Schafer R, Handschel J, Franz M, et al. Effects of an occlusal splint compared with cognitive-behavioral treatment on sleep bruxism activity. European Journal of Oral Sciences 2007;115(1):7-14. [DOI: 10.1111/j.1600-0722.2007.00417.x] [DOI] [PubMed] [Google Scholar]
Pfeil 1987 {published data only}
- Pfeil AT. A cognitive-behavioral group intervention for patients with chronic temporomandibular joint pan: an exploratory study. Proquest Dissertations and Theses 1987.
Takeuchi Sato 2019 {published data only}
- Takeuchi-Sato T, Ono Y, Funato M, Sato H, Suganuma T, Baba K. Efficacy of an email-based recording and reminding system for limiting daytime nonfunctional tooth contact in patients with temporomandibular disorders: a randomized controlled trial. Journal of Oral Rehabilitation 2019;47(2):158-63. [DOI: 10.1111/joor.12875] [DOI] [PubMed] [Google Scholar]
Treacy 1999 {published data only}
- Treacy K. Awareness/relaxation training and transcutaneous electrical neural stimulation in the treatment of bruxism. Journal of Oral Rehabilitation 1999;26(4):280-7. [DOI: 10.1046/j.1365-2842.1999.00381.x] [DOI] [PubMed] [Google Scholar]
van Grootel 2017 {published data only}
- Grootel RJ, Buchner R, Wismeijer D, Glas HW. Towards an optimal therapy strategy for myogenous TMD, physiotherapy compared with occlusal splint therapy in an RCT with therapy-and-patient-specific treatment durations. BMC Musculoskeletal Disorders 2017;18(1):76. [DOI: 10.1186/s12891-017-1404-9] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to ongoing studies
CTRI2007091000047 {published data only}
- CTRI/2007/091/000047. A clinical trial to study the effects of three physiotherapy treatments - jaw muscle exercise, myofascial release and cognitive behavioral therapy in temporomandibular muscle pain patients. www.who.int/trialsearch/Trial2.aspx?TrialID=CTRI/2007/091/000047 (first received 18 January 2008).
JPRN‐C000000365 {published data only}
- JPRN-C000000365. Randomized controlled clinical trial of cognitive behavioral therapy for chronic muscle pain in head, neck and shoulder regions. www.who.int/trialsearch/Trial2.aspx?TrialID=JPRN-C000000365 (first received 1 March 2006).
NCT00067366 {published data only}
- NCT00067366. Brief treatment for temporomandibular pain. clinicaltrials.gov/show/NCT00067366 (first received 19 August 2003).
NCT00769561 {published data only}
- NCT00769561. Biofeedback-based cognitive behavioral treatment for temporomandibular disorders. clinicaltrials.gov/show/NCT00769561 (first received 9 October 2008).
NCT04363762 {published data only}
- NCT04363762. An internet-based multimodal pain program for chronic temporomandibular disorder pain. clinicaltrials.gov/show/NCT04363762 (first received 27 April 2020).
Additional references
AAOP 1996
- American Academy of Orofacial Pain. Orofacial pain: Guidelines for assessment, diagnosis and management. Chicago: Quintessence, 1996. [Google Scholar]
Aggarwal 2010
- Aggarwal VR, Tickle M, Javidi H, Peters S. Reviewing the evidence: can cognitive behavioral therapy improve outcomes for patients with chronic orofacial pain? Journal of Orofacial Pain 2010;24:163-71. [PubMed] [Google Scholar]
Aggarwal 2012
- Aggarwal VR, Joughin A, Zakrzewska J, Appelbe P, Tickle M. Dentists' preferences for diagnosis, management and referral of chronic oro-facial pain: results from a national survey. Health Education Journal 2012;71(6):662-9. [Google Scholar]
Al‐Baghdadi 2014
- Al-Baghdadi M, Durham J, Araujo-Soares V, Robalino S, Errington L, Steele J. TMJ disc displacement without reduction management: a systematic review. Journal of Dental Research 2014;93(7 Suppl):37S-51S. [DOI] [PMC free article] [PubMed] [Google Scholar]
Andreu 2006
- Andreu Y, Galdón MJ, Durá E, Ferrando M, Pascual J, Turk DC, et al. An examination of the psychometric structure of the Multidimensional Pain Inventory in temporomandibular patients. Head & Face Medicine 2006;2:48-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
Barker 2019
- Barker S, Urbanek M, Penlington C. Psychological interventions for persistent orofacial pain. Primary Dental Journal 2019;7(4):30-5. [PubMed] [Google Scholar]
Beck 1988
- Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring clinical anxiety: psychometric properties. Journal of Consulting and Clinical Psychology 1988;56(6):893-7. [DOI] [PubMed] [Google Scholar]
Beck 1996
- Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory-II. San Antonio, TX: Psychological Corporation, 1996. [Google Scholar]
Beecroft 2019
- Beecroft E, Penlington C, Desai H, Durham J. Temporomandibular disorder for the general dental practitioner. Primary Dental Journal 2019;7(4):62-70. [PubMed] [Google Scholar]
Bell 2020
- Bell L, Cornish P, Gauthier R, Kargus C, Rash J, Robbins R, et al. Implementation of the Ottawa Hospital Pain Clinic stepped care program: a preliminary report. Canadian Journal of Pain 2020;4(1):168-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bower 2005
- Bower P, Gilbody S. Stepped care in psychological therapies: access, effectiveness and efficiency. British Journal of Psychiatry 2005;186(1):11-7. [DOI] [PubMed] [Google Scholar]
Bush 1995
- Bush FM, Dolwick MF. The Temporomandibular Joint and Related Orofacial Disorders. Philadelphia: Lippincott, 1995. [Google Scholar]
Christidis 2019
- Christidis N, Lindström Ndanshau EN, Sandberg A, Tsilingaridis G. Prevalence and treatment strategies regarding temporomandibular disorders in children and adolescents: a systematic review. Journal of Oral Rehabilitation 2019;46(3):291-301. [DOI] [PubMed] [Google Scholar]
Cohen 1983
- Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. Journal of Health and Social Behavior 1983;24(4):385-96. [PubMed] [Google Scholar]
Dahl 2004
- Dahl JA, Wilson KG, Nilsson A. Acceptance and commitment therapy and the treatment of persons at risk for long-term disability resulting from stress and pain symptoms: a preliminary randomized trial. Behavior Therapy 2004;35(4):785-801. [Google Scholar]
De Leeuw 2008
- De Leeuw R, Klasser GD. Orofacial Pain: Guidelines for Assessment, Diagnosis, and Management. Chicago: Quintessence Books, 2008. [Google Scholar]
Derogatis 1983
- Derogatis LR, Melisaratos N. The brief symptom inventory: an introductory report. Psychological Medicine 1983;13(3):595-605. [PubMed] [Google Scholar]
Durham 2007
- Durham J, Exley C, Wassell R, Steele JG. ‘Management is a black art'–professional ideologies with respect to temporomandibular disorders. British Dental Journal 2007;202(11):E29. [DOI] [PubMed] [Google Scholar]
Durham 2010
- Durham J, Steele JG, Wassell RW, Exley C. Living with uncertainty: temporomandibular disorders. Journal of Dental Research 2010;89(8):827-30. [DOI] [PubMed] [Google Scholar]
Durham 2015
- Durham J. Summary of Royal College of Surgeons' (England) clinical guidelines on management of temporomandibular disorders in primary care. British Dental Journal 2015;218(6):355-6. [DOI] [PubMed] [Google Scholar]
Dworkin 1992
- Dworkin SF, LeResche L. Research diagnostic criteria for temporomandibular disorders: review, criteria, examinations and specifications, critique. Journal of Craniomandibular Disorders: Facial and Oral Pain 1992;6(4):301-55. [PubMed] [Google Scholar]
Dworkin 2005
- Dworkin RH, Turk DC, Farrar JT, Haythornthwaite JA. Core outcome measures for chronic pain clinical trials: IMMPACT recommendations. Pain 2005;113(1):9-19. [DOI] [PubMed] [Google Scholar]
Farrar 2001
- Farrar JT. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical rating scale. Pain 2001;94(2):149-58. [DOI] [PubMed] [Google Scholar]
Fordyce 1968
- Fordyce WE, Fowler RS Jr, Lehmann JF, DeLateur BJ. Some implications of learning in problems of chronic pain. Journal of Chronic Diseases 1968;21(3):179-90. [DOI] [PubMed] [Google Scholar]
Gatchel 2014
- Gatchel RJ, Kishino ND, Schultz IZ. Early intervention to prevent the development of chronic musculoskeletal pain disorders and disability. In: Handbook of Musculoskeletal Pain and Disability Disorders in the Workplace. New York (NY): Springer, 2014:379-93. [Google Scholar]
Goldberg 1977
- Goldberg D, Kay C, Thompson L. Psychiatric morbidity in general practice and the community. Psychological Medicine 1977;6(4):565-9. [DOI] [PubMed] [Google Scholar]
Gooding 2020
- Gooding H, Stedmon J, Crix D. ‘All these things don't take the pain away but they do help you to accept it': making the case for compassion-focused therapy in the management of persistent pain. British Journal of Pain 2020;14(1):31-41. [DOI: 10.1177/2049463719857099] [DOI] [PMC free article] [PubMed]
GRADEpro GDT [Computer program]
- GRADEpro GDT. Version accessed 6 November 2019. Hamilton, ON: McMaster University (developed by Evidence Prime). Available at gradepro.org.
Greene 2010
- Greene CS. Managing the care of patients with temporomandibular disorders. Journal of the American Dental Association 2010;141(9):1086-8. [DOI] [PubMed] [Google Scholar]
Hayes 1999
- Hayes SC, Strosahl K, Wilson KG. Acceptance and Commitment Therapy: an Experiential Approach to Behavior Change. New York: Guildford Press, 1999. [Google Scholar]
Higgins 2017
- Higgins JP, Altman DG, Sterne JA, editor(s). Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Churchill R, Chandler J, Cumpston MS, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.2.0 (updated June 2017). Cochrane, 2017. Available from www.training.cochrane.org/handbook/archive/v5.2.
Higgins 2019a
- Higgins JP, Eldridge S, Li T, editor(s). Chapter 23: Including variants on randomized trials. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.0 (updated July 2019). Cochrane, 2019. Available from www.training.cochrane.org/handbook.
Higgins 2019b
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions. 2nd edition. Chichester (UK): John Wiley & Sons, 2019. [Google Scholar]
Hill 2011
- Hill JC, Whitehurst DG, Lewis M, Bryan S, Dunn KM, Foster NE, et al. Comparison of stratified primary care management for low back pain with current best practice (STarT Back): a randomised controlled trial. Lancet 2011;378(9802):1560-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kabat‐Zinn 1982
- Kabat-Zinn J. An outpatient program in behavioral medicine for chronic pain patients based on the practice of mindfulness meditation: theoretical considerations and preliminary results. General Hospital Psychiatry 1982;4(1):33-47. [DOI] [PubMed] [Google Scholar]
Kerns 1985
- Kerns RD, Turk DC, Rudy TE. The West Haven-Yale Multidimensional Pain Inventory. Pain 1985;23:345-56. [DOI] [PubMed] [Google Scholar]
Kotiranta 2014
- Kotiranta U, Suvinen T, Forssell H. Tailored treatments in temporomandibular disorders: where are we now? A systematic qualitative literature review. Journal of Oral and Facial Pain and Headache 2014;28:28-37. [DOI] [PubMed] [Google Scholar]
Kroenke 2001
- Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. Journal of General Internal Medicine 2001;16(9):606-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kroenke 2002
- Kroenke K, Spitzer RL, Williams JBW. The PHQ-15: validity of a new measure for evaluating the severity of somatic symptoms. Psychosomatic Medicine 2002;64(2):258-66. [DOI] [PubMed] [Google Scholar]
Kroenke 2009
- Kroenke K, Spitzer RL, Williams JB, Löwe B. An ultra-brief screening scale for anxiety and depression: the PHQ–4. Psychosomatics 2009;50(6):613-21. [DOI] [PubMed] [Google Scholar]
Lefebvre 2020
- Lefebvre C, Glanville J, Briscoe S, Littlewood A, Marshall C, Metzendorf M-I, et al. Technical Supplement to Chapter 4: Searching for and selecting studies. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.1 (updated September 2020). Cochrane, 2020. Available from www.training.cochrane.org/handbook.
Liu 2012
- Liu HX, Liang QJ, Xiao HX, Gao Y, Ahmetjiang A. The effectiveness of cognitive-behavioural therapy for temporomandibular disorders: a systematic review. Journal of Oral Rehabilitation 2012;39:55-62. [DOI] [PubMed] [Google Scholar]
Loeser 1999
- Loeser JD, Melzack R. Pain: an overview. Lancet 1999;353(9164):1607-9. [DOI] [PubMed] [Google Scholar]
McNair 1971
- McNair D, Lorr M, Droppleman LF. Profile of Mood States. San Diego: Educational and Industrial Testing Service, 1971. [Google Scholar]
Melzack 1975
- Melzack R. The McGill Pain Questionnaire: major properties and scoring methods. Pain 1975;1(3):277-99. [DOI] [PubMed] [Google Scholar]
Mujakperuo 2010
- Mujakperuo HR, Watson M, Morrison R, Macfarlane TV. Pharmacological interventions for pain in patients with temporomandibular disorders. Cochrane Database of Systematic Reviews 2010, Issue 10. Art. No: CD004715. [DOI: 10.1002/14651858.CD004715.pub2] [DOI] [PubMed] [Google Scholar]
NICE 2016
- National Institute for Health and Care Excellence (NICE). Clinical knowledge summary: temporomandibular disorders (TMDs). Last revised December 2016. Available at cks.nice.org.uk/temporomandibular-disorders-tmds (accessed 16 November 2019).
Nicholas 2007
- Nicholas MK. The pain self-efficacy questionnaire: taking pain into account. European Journal of Pain 2007;11(2):153-63. [DOI] [PubMed] [Google Scholar]
NIDCR 2019
- National Institute of Dental and Craniofacial Research (NIDCR). Facial pain. nidcr.nih.gov/research/data-statistics/facial-pain (accessed 20 September 2019).
Nilsson 2007
- Nilsson I, List T, Drangsholt M. Incidence and temporal patterns of temporomandibular disorder pain among Swedish adolescents. Journal of Orofacial Pain 2007;21(2):127-32. [PubMed] [Google Scholar]
O'Sullivan 2012
- O'Sullivan P. It's time for change with the management of non-specific chronic low back pain. British Journal of Sports Medicine 2012;46(4):224-7. [DOI] [PubMed] [Google Scholar]
Ohrbach 2018
- Ohrbach R, Durham J. Biopsychosocial aspects of orofacial pain. In: Farah CS, Balasubramaniam R, McCullough MJ, editors(s). Contemporary Oral Medicine. Heidelberg: Springer Meteor, 2018:1-21. [Google Scholar]
Okifuji 1999
- Okifuji A, Turk DC, Eveleigh DJ. Improving the rate of classification of patients with the multidimensional pain inventory (MPI): clarifying the meaning of "significant other". Clinical Journal of Pain 1999;15(4):290-6. [DOI] [PubMed] [Google Scholar]
Oppe 2014
- Oppe M, Devlin NJ, Hout B, Krabbe PF, Charro F. A program of methodological research to arrive at the new international EQ-5D-5L valuation protocol. Value in Health 2014;17(4):445-53. [DOI] [PubMed] [Google Scholar]
Paço 2016
- Paço M, Peleteiro B, Duarte J, Pinho T. The effectiveness of physiotherapy in the management of temporomandibular disorders: a systematic review and meta-analysis. Journal of Oral & Facial Pain and Headache 2016;30(3):210-20. [DOI] [PubMed] [Google Scholar]
Palmer 2022
Penlington 2019a
- Penlington C. Exploring a compassion-focused intervention for persistent pain in a group setting. British Journal of Pain 2019;13(1):59-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
Penlington 2019b
- Penlington C, Urbanek M, Barker S. Psychological theories of pain. Primary Dental Journal 2019;7(4):24-9. [PubMed] [Google Scholar]
Peters 2015
- Peters S, Goldthorpe J, McElroy C, King E, Javidi H, Tickle M, et al. Managing chronic orofacial pain: a qualitative study of patients', doctors', and dentists' experiences. British Journal of Health Psychology 2015;20(4):777-91. [DOI] [PubMed] [Google Scholar]
Radloff 1977
- Radloff LS. The CES-D Scale: A self-report depression scale for research in the general population. Applied Psychological Measurement 1977;1:385-401. [Google Scholar]
Randhawa 2016
- Randhawa K, Bohay R, Côté P, Velde G, Sutton D, Wong JJ, et al. The effectiveness of noninvasive interventions for temporomandibular disorders: a systematic review by the Ontario protocol for traffic injury management (OPTIMa) collaboration. Clinical Journal of Pain 2016;32(3):260-78. [DOI] [PubMed] [Google Scholar]
Review Manager 2020 [Computer program]
- Review Manager 5 (RevMan 5). Version 5.4. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2020.
Roland 2000
- Roland M, Fairbank J. The Roland–Morris Disability Questionnaire and the Oswestry Disability Questionnaire. Spine 2000;25(24):3115-24. [DOI] [PubMed] [Google Scholar]
Schiffman 2014
- Schiffman E, Ohrbach R, Truelove E, Look J, Anderson G, Goulet J, et al. Diagnostic criteria for temporomandibular disorders (DC/TMD) for clinical and research applications: recommendations of the International RDC/TMD Consortium Network and Orofacial Pain Special Interest Group. Journal of Oral & Facial Pain and Headache 2014;28(1):6-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schünemann 2017
- Schünemann HJ, Wiercioch W, Brozek J, Etxeandia-Ikobaltzeta I, Mustafa RA, Manja V, et al. GRADE Evidence to Decision (EtD) frameworks for adoption, adaptation and de novo development of trustworthy recommendations: GRADE ADOLOPMENT. Journal of Clinical Epidemiology 2017;81:101-10. [DOI] [PubMed] [Google Scholar]
Sharma 2018
- Sharma S, Ohrbach R. Definition, aetiology, and epidemiology of temporomandibular disorders. In: Fernández-de-las-Peñas C, Mesa J, editors(s). Temporomandibular Disorders: Manual Therapy, Exercise and Needling Therapies. Edinburgh: Handspring Publishing, 2018. [Google Scholar]
Singh 2017
- Singh BP, Jayaraman S, Kirubakaran R, Joseph S, Muthu MS, Jivnani H, et al. Occlusal interventions for managing temporomandibular disorders. Cochrane Database of Systematic Reviews 2017, Issue 11. Art. No: CD012850. [DOI: 10.1002/14651858.CD012850] [DOI] [PMC free article] [PubMed] [Google Scholar]
Slade 1994
- Slade GD, Spencer AJ. Development and evaluation of the oral health impact profile. Community Dental Health 1994;11(1):3-11. [PubMed] [Google Scholar]
Slade 2013
- Slade GD, Fillingim RB, Sanders AE, Bair E, Greenspan JD, Ohrbach R, et al. Summary of findings from the OPPERA prospective cohort study of incidence of first-onset temporomandibular disorder: implications and future directions. Journal of Pain 2013;14(12 Suppl):T116-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Slade 2016
- Slade GD, Ohrbach R, Greenspan JD, Fillingim RB, Bair E, Sanders AE, et al. Painful temporomandibular disorder: decade of discovery from OPPERA studies. Journal of Dental Research 2016;95(10):1084-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
Smith 2020
- Smith SM, Dworkin RH, Turk DC, McDermott MP, Eccleston C, Farrar JT, et al. Interpretation of chronic pain clinical trial outcomes: IMMPACT recommended considerations. Pain 2020;16(11):2446-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Story 2016
- Story WP, Durham J, Al-Baghdadi M, Steele J, Araujo-Soares V. Self-management in temporomandibular disorders: a systematic review of behavioural components. Journal of Oral Rehabilitation 2016;43(10):759-70. [DOI] [PubMed] [Google Scholar]
Tan 2004
- Tan G, Jensen MP, Thornby JI, Shanti BF. Validation of the Brief Pain Inventory for chronic nonmalignant pain. The Journal of Pain 2004;5(2):133-7. [DOI] [PubMed] [Google Scholar]
Treede 2015
- Treede RD, Rief W, Barke A, Aziz Q, Bennett MI, Benoliel R, et al. A classification of chronic pain for ICD-11. Pain 2015;156(6):1003-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Turk 1983
- Turk DC, Meichenbaum D, Genest M. Pain and Behavioral Medicine: a Cognitive-behavioral Perspective. 1st edition. New York (NY): Guilford Press, 1983. [Google Scholar]
Turner 2007
- Turner JA, Holtzman S, Lloyd M. Mediators, moderators, and predictors of therapeutic change in cognitive–behavioral therapy for chronic pain. Pain 2007;127(3):276-86. [DOI] [PubMed] [Google Scholar]
Von Korff 1992
- Von Korff M, Ormel J, Keefe FJ, Dworkin SF. Grading the severity of chronic pain. Pain 1992;50(2):133-49. [DOI] [PubMed] [Google Scholar]
Ware 1994
- Ware JE, Kosinski M, Keller SD. SF-36 Physical and mental health summary scales: A user's manual. Boston: Health Assessment Lab, 1994. [Google Scholar]
Williams 2012
- Williams AC, Eccleston C, Morley S. Psychological therapies for the management of chronic pain (excluding headache) in adults. Cochrane Database of Systematic Reviews 2012, Issue 11. Art. No: CD007407. [DOI: 10.1002/14651858.CD007407.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Williams 2020
- Williams AC de C, Fisher E, Hearn L, Eccleston C. Psychological therapies for the management of chronic pain (excluding headache) in adults. Cochrane Database of Systematic Reviews 2020, Issue 8. Art. No: CD007407. [DOI: 10.1002/14651858.CD007407.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zigmond 1983
- Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatrica Scandinavica 1983;67(6):361-70. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Penlington 2019c
- Penlington C, Otemade AA, Bowes C, Taylor G, Waterhouse P, Ohrbach R. Psychological therapies for temporomandibular disorders (TMD). Cochrane Database of Systematic Reviews 2019, Issue 12. Art. No: CD013515. [DOI: 10.1002/14651858.CD013515] [DOI] [PMC free article] [PubMed] [Google Scholar]


