Scheme 8.
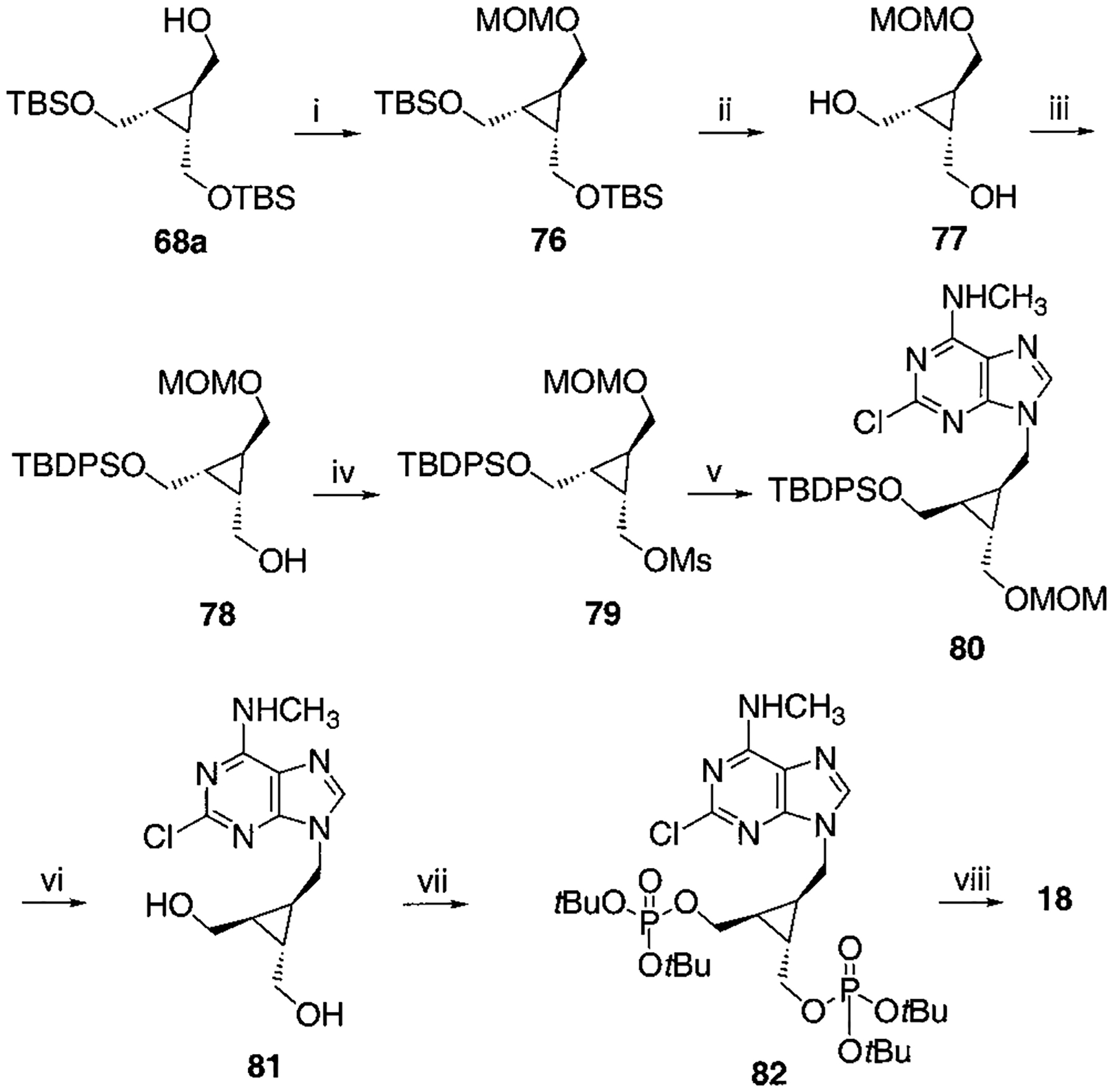
Synthesis of Racemic Cyclopropyl Bisphosphate Derivative, Compound 18a
a Reaction conditions: (i) CH3OCH2Cl, Hünig base, CH2Cl2, rt, 4 h, 93%; (ii) TBAF, THF, rt, 3 h, 95%; (iii) TBDPSCl (1 equiv), DMAP (1 equiv), CH2Cl2, rt, 3 d, 70%; (iv) MsCl, TEA, CH2Cl2, rt, 1 h, 95%; (v) 2-chloro-N6-methylaminopurine, K2CO3, DMF, 70 °C, overnight, 71%; (vi) c-HCl:MeOH = 1:3, 80 °C, 4 h, 65%; (vii) Et2NP(OtBu)2, tetrazole, THF, rt, 20 min then MCPBA, 62%; (viii) 5% TFA in CH2Cl2, rt, 30 min, 71%.
