Scheme 2.
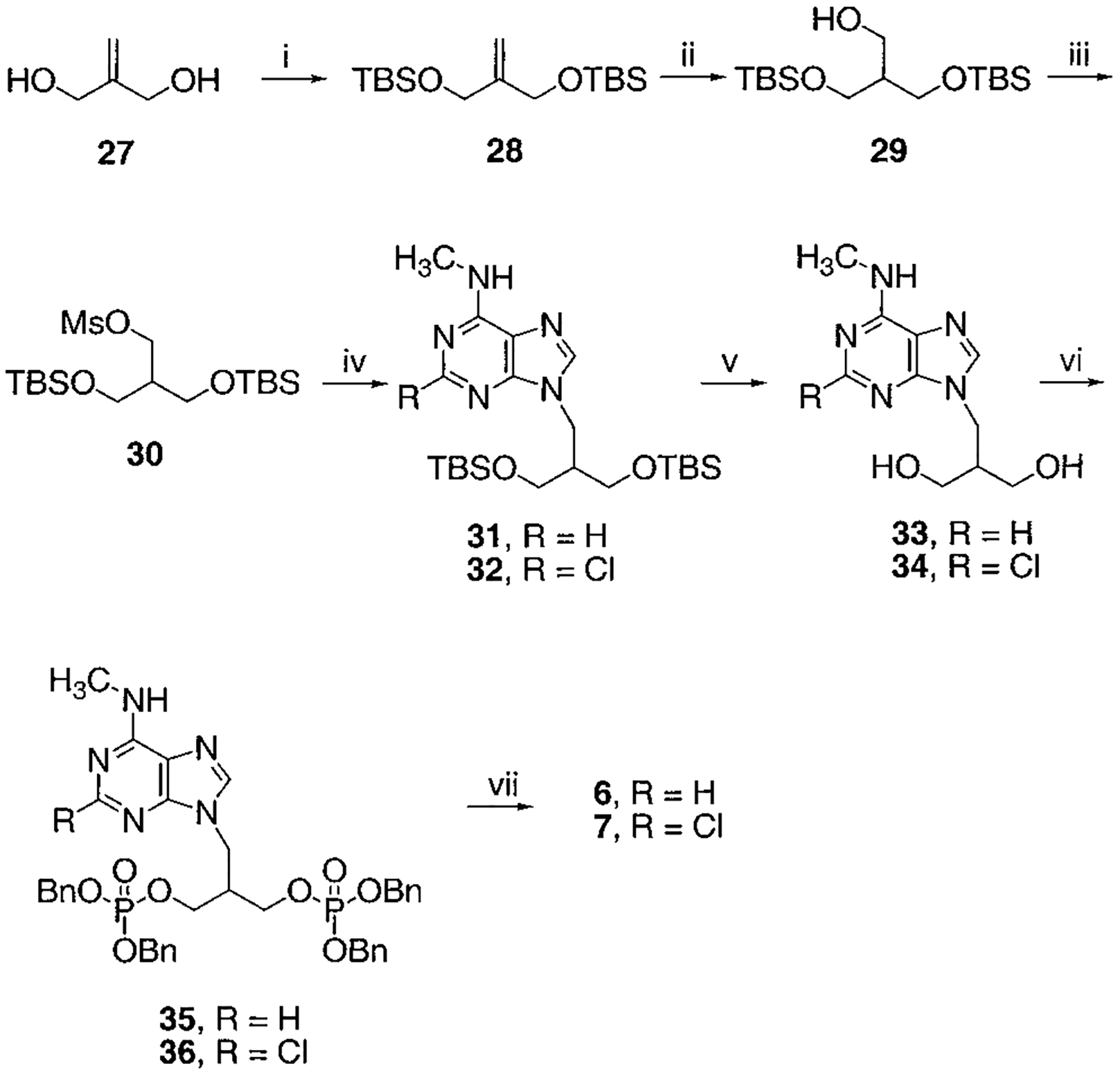
Synthesis of Isobutyl Bisphosphates, Compounds 6 and 7a
a Reaction conditions: (i) TBSCl, TEA, DMAP, CH2Cl2, rt, overnight, 99%; (ii) BH3, THF, rt, overnight, then H2O2, NaOH, 60%; (iii) MsCl, TEA, CH2Cl2, rt, 1 h; (iv) 2-chloro- N6-methylaminopurine (for 32) or N6-methylaminopurine (for 31), K2CO3, 18-crown-6, DMF, 60% two-step yield for 31, 72% two-step yield for 32; (v) AcOH:H2O:THF = 3:2:2, rt, 2 d, 79% for 33, 93% for 34; (vi) tetrabenzylpyrophosphate, NaH, THF, rt, 1 h, 81% for 35, 53% for 36; (vii) BCl3, CH2Cl2, 5 °C, 2 d, 75% for 6, 41% for 7.
