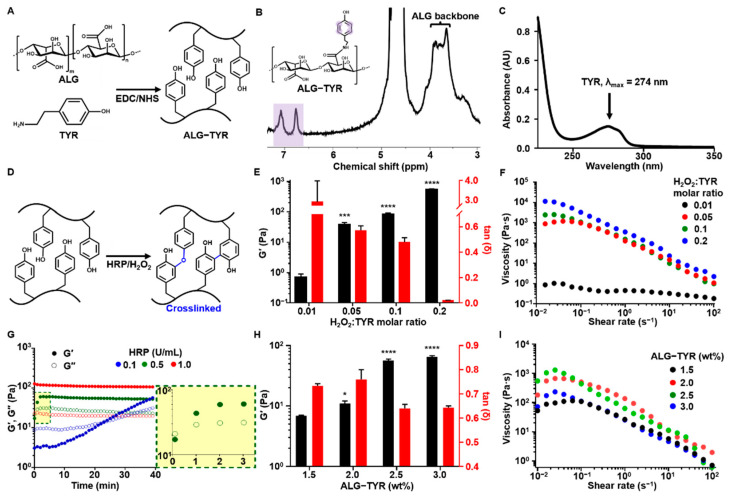
Figure 2.
The ALG-TYR hydrogel characterization. (A) The ALG-TYR conjugation mechanism with the EDC/NHS coupling reaction. The carboxyl group in the alginate binds to the amine group of the tyramine to form a phenol-functionalized backbone chain. (B) The 1H NMR analysis of ALG-TYR. Different marked peaks (purple) represent hydrogen atoms in tyramine. (C) UV–Vis spectroscopy at the 274 nm peak (tyramine). (D) The enzymatic crosslinking mechanism of ALG-TYR chains induced by HRP/H2O2. (E) The storage modulus and tan δ of different H2O2:TYR ratios at 1% strain and 1 Hz. (F) The changes in viscosity depend on the applied shear rate on different H2O2:TYR ratios. The significance of each G’ data was compared with that of the H2O2:TYR ratio at 0.01. (G) The gelation time kinetics observed through changes in the HRP concentrations. Highlighted region: 0.5 U/mL HRP. (H) The storage modulus and tan δ of different concentrations of ALG-TYR at 1% strain and 1 Hz. The significance of each G’ data was compared with that of 1.5% ALG-TYR. (I) Changes in viscosity depend on the applied shear rate on different concentrations of ALG-TYR. (n = 3, mean ± SD) (* p < 0.05, *** p < 0.001, **** p < 0.0001).