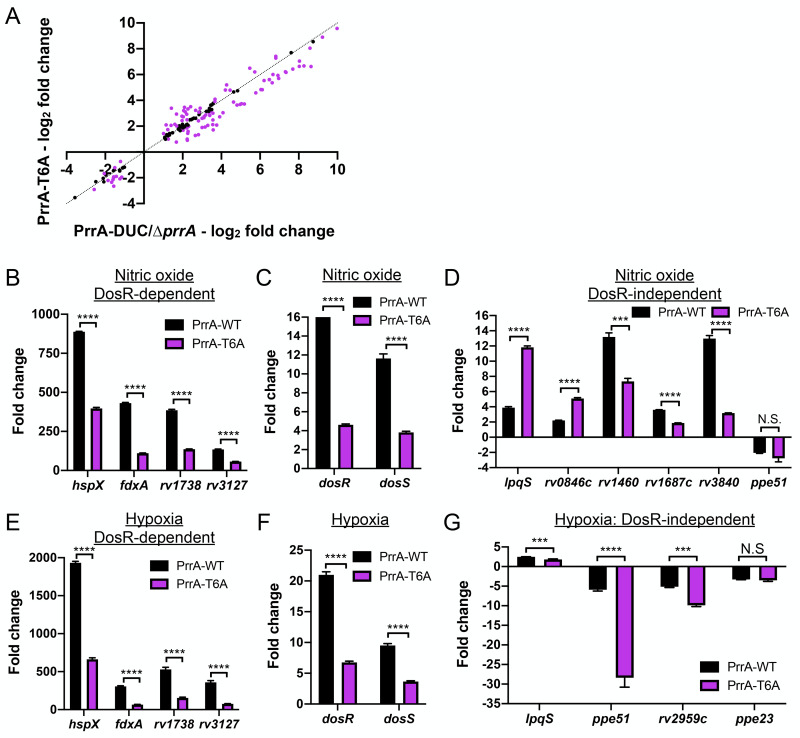
Fig 6. STPK phosphorylation of PrrA is critical for Mtb response to NO and hypoxia.
(A-D) A STPK-phosphoablative PrrA variant strongly affects Mtb response to NO. PrrA-DUC/ΔprrA and PrrA-T6A-DUC/ΔprrA (“PrrA-T6A”) Mtb were grown in 7H9, pH 7.0 ± 100 μM DETA NONOate for four hours. RNA was extracted for RNA sequencing analysis (A) or qRT-pCR (B-D). In (A), log2-fold change compares gene expression in the 7H9, pH 7.0 + 100 μM DETA NONOate condition versus the 7H9, pH 7.0 control condition. Genes marked in purple had a log2-fold change difference ≥0.25 between the PrrA-T6A-DUC/ΔprrA and PrrA-DUC/ΔprrA strains (p<0.05, FDR<0.01 in both sets, with log2-fold change ≥1 in the PrrA-DUC/ΔprrA set). In (B-D), fold change compares the 7H9, pH 7 + 100 μM DETA NONOate condition versus the 7H9, pH 7 control condition. sigA was used as the control gene, and data are shown as means ± SD from 3 technical replicates. p-values were obtained with an unpaired t-test. N.S. not significant, *** p<0.001, **** p<0.0001. (E-G) The PrrA-T6A variant strain modulates Mtb response to hypoxia. qRT-PCR of PrrA-DUC/ΔprrA (“PrrA-WT”) and PrrA-T6A-DUC/ΔprrA (“PrrA-T6A”) were grown under aerated conditions before exposure to 1% oxygen for four hours. Fold change compares the 1% oxygen condition at 4 hours to the aerated 0 hour time point. sigA was used as the control gene, and data are shown as means ± SD from 3 technical replicates. p-values were obtained with an unpaired t-test. N.S. not significant, *** p<0.001, **** p<0.0001.