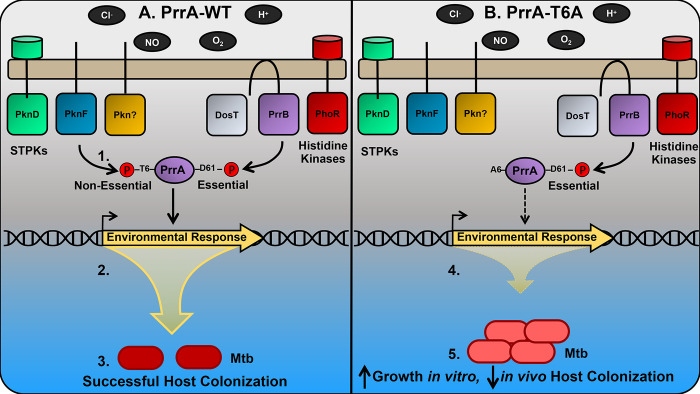
Fig 9. Schematic model of environmental signal integration via PrrA in Mtb.
A schematic model illustrating signal integration and outcome in the presence of WT PrrA (A) versus a STPK-phosphoablative PrrA-T6A variant (B) is shown. 1. In addition to the cognate HK PrrB, other HKs are predicted to interact with PrrA [64], while several STPKs are predicted to phosphorylate PrrA [29, 48]. Phosphorylation of PrrA by STPKs is non-essential for Mtb viability in rich broth. In contrast, HK-mediated phosphorylation of PrrA is essential for Mtb viability in rich broth, even though PrrB is non-essential. 2. PrrA contributes to the control of Mtb transcriptional response to acidic pH, Cl-, nitric oxide (NO), and hypoxia (low O2), with PrrA function regulated by both STPK- and HK-mediated phosphorylation. 3. Appropriate environmental response via WT PrrA results in adaptation, proper Mtb growth control, and successful host colonization. 4. When STPK-mediated phosphorylation of PrrA is prevented at the T6 residue (PrrA-T6A variant), appropriate transcriptional response to pH, Cl-, NO, and hypoxia is prevented (predominantly dampened). 5. The disrupted transcriptional response consequently results in failure of Mtb to adapt to its local environment, with the failure to respond to growth-inhibiting cues increasing Mtb growth in vitro and attenuating the bacterium’s ability to colonize the host in vivo.