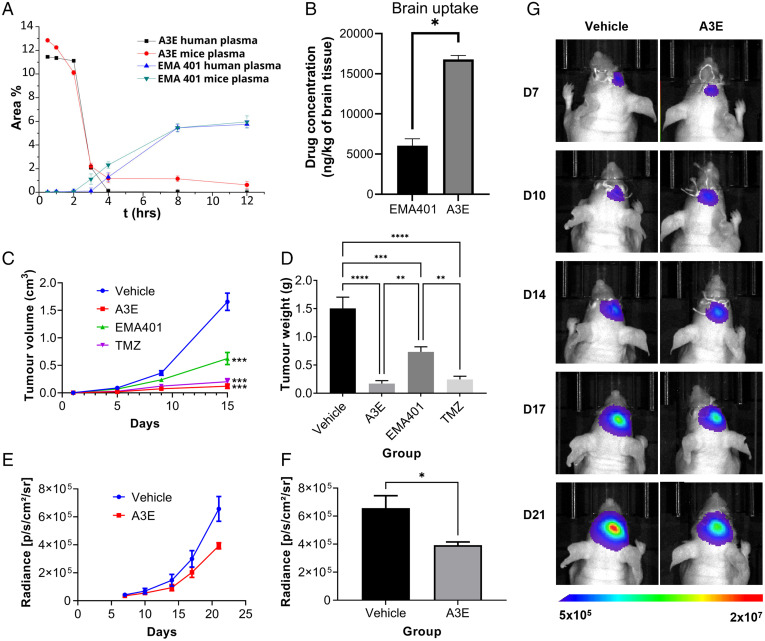
Fig. 8.
Stability and efficacy of EMA401 and A3E in vivo. (A) Plasma-stability profile of A3E after incubation in human and mouse plasma. (B) Brain biodistribution of EMA401 after intravenous injection of EMA401 (10 mg/kg; n = 3) and A3E (containing 10 mg/kg of EMA401; n = 3) measured in brains of C57BL/6 mice. Average (C) volume and (D) weight of subcutaneous U87-GFP/luc tumors in NSG mice after 21 d of treatment with EMA401 (10 mg/kg; n = 9), A3E (25.88 mg/kg; n = 8), TMZ (30 mg/kg; n = 9), or vehicle (PBS with 5% dimethyl sulfoxide [DMSO] and 5% solutol; n = 7). (E and F) Bioluminescent imaging data of intracranial U87-GFP/luc tumors in BALB/c nude mice treated with A3E (25.88 mg/kg; n = 6) or vehicle (PBS with 5% DMSO and 5% solutol; n = 6). (G) Representative bioluminescent images of one mouse in each treatment group at each time point. Data represent the mean ± SEM. Statistical analysis was conducted using one-way or two-way ANOVA with the Tukey’s test or Student’s t test. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001, ****P ≤ 0.0001.