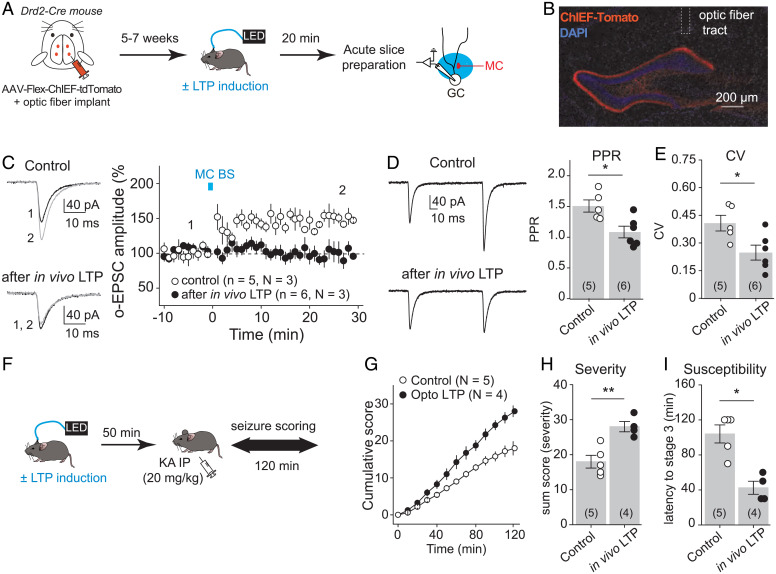
Fig. 6.
In vivo induction of MC-GC LTP promoted seizures. (A) Experimental timeline. To selectively photostimulate MC axons in vivo, AAV-hSyn-ChIEF-tdTomato was bilaterally injected into ventral and dorsal DG of Drd2-cre mice, and an optical fiber was implanted above the IML of the DG. The LTP induction protocol (MC BS: five pulses at 30 Hz, repeated 50 times, every 0.5 s) was applied in vivo by delivering blue light through a patch cord cable connected to a fiber-coupled 470-nm light-emitting diode (LED) source 5 to 7 wk after surgery. Sham light was used as a control. Acute hippocampal slices were prepared 20 min later, and MC-GC synaptic properties were analyzed using whole-cell recordings of GCs and light stimulation of MC axons. (B) Representative confocal images showing the viral expression and the fiber tract location. (C) Optically evoked EPSCs (o-EPSCs) were recorded in GC in response to MC axon photostimulation. Representative traces (light) and time course summary plot (light) showing that in vivo application of MC BS prevented subsequent induction of LTP in vitro. (D and E) PPR (D) and CV (E) were both significantly decreased after in vivo application of the MC-GC LTP induction protocol as compared with the sham light (control) condition. Each number in parentheses indicates the number of cells. (F) Experimental timeline. The LTP induction protocol (MC BS: five pulses at 30 Hz, repeated 50 times, every 0.5 s) was delivered in vivo, while sham light was used as control. KA (20 mg/kg IP) was then administered 50 min after the optogenetic stimulation, and seizures were scored for 120 min. (G–I) Application of the MC-GC LTP induction protocol (optogenetically-induced LTP or Opto LTP) increased seizure severity and susceptibility, as indicated by significant increases in the cumulative seizure score (G) and in the sum score (H) and a significantly decrease in latency to convulsive seizures (I). *P < 0.05, **P < 0.01. Data are presented as mean ± SEM.