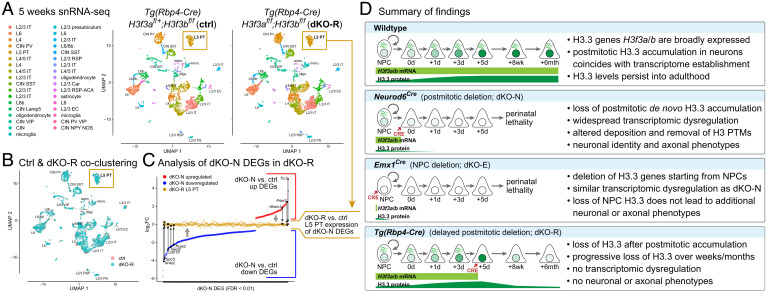
Fig. 7.
Transcriptome maintenance following H3f3a and H3f3b codeletion after initial H3.3 accumulation. (A and B) snRNA-seq of control and dKO-R cortex at postnatal 5 wk visualized by UMAP. Clustering of ctrl (salmon) and dKO-R (turquoise) cells showed the same 34 cell clusters in UMAP space (A) with substantial overlap of each cluster (B), including the L5 PT neurons (gold boxes) in which Tg(Rbp4-Cre) is active. (C) DEGs from dKO-N were compared based on log2FC in dKO-N and in dKO-R L5 PT neurons. Both up (red) and down (blue) regulated dKO-N DEGs showed normalization toward log2FC of 0 (no change) in dKO-R L5 PT neurons (gold). (D) Summary of key findings. Cortical neurons undergo substantial de novo H3.3 accumulation postmitosis. Postmitotic H3.3 is required for developmental establishment of neuronal chromatin and transcriptome, acquisition of distinct neuronal identities, and formation of axon tracts. The first few postmitotic days are a critical window for de novo H3.3 in neurons. This early H3.3 accumulation differs in timescale from long-term H3.3 turnover, which occurs over weeks to months.