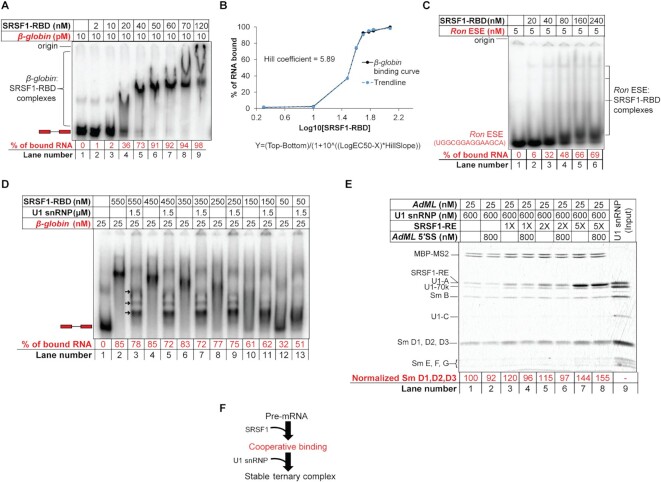
Figure 2.
Cooperative binding of SRSF1-RBD to β-globin is important for stabilization of U1 snRNP. (A) EMSA showing gradual upshift of radiolabeled β-globin complexes upon titration with SRSF1-RBD. (B) Binding curve (solid black line) of SRSF1-RBD to β-globin and Hill coefficient obtained from band intensity of the free probe in (A); the equation of the curve is given below it, where Y is band intensity, X is log10[SRSF1], and top and bottom are plateaus in the unit of the Y axis; a trendline (broken blue line) was generated to visually examine the goodness of fit by estimating the Y values from the Xvalues, Hill slope (5.89), EC50 (33), top (98.18) and bottom (1.85). (C) EMSA showing formation of weak complexes with a radiolabeled 14-nt long RNA containing Ron ESE (a binding site for SRSF1). (D) Binding efficiency of SRSF1-RBD diminishes with decreasing SRSF1:β-globin ratio below 10:1 (compare lanes 8 and 10) with a corresponding decline in the ternary complex formation with U1 snRNP (compare lanes 9 and 11). (E) Amylose pull-down assay of AdML (25 nM) bound to MBP-MS2 showing enhanced stability of U1 snRNP with 5× molar excess SRSF1-RE (125 nM) compared to 2×, 1× or no SRSF1-RE, particularly when challenged with a 14-nt long RNA containing AdML 5′SS; Sm D1, D2, D3 band intensity is normalized to MBP-MS2 level. (F) Summary flow chart: Specific U1 snRNP recruitment requires cooperative binding of SRSF1, which in turn requires a threshold level of SRSF1:pre-mRNA molar ratio; red text indicates the step added based on the conclusions of this figure.