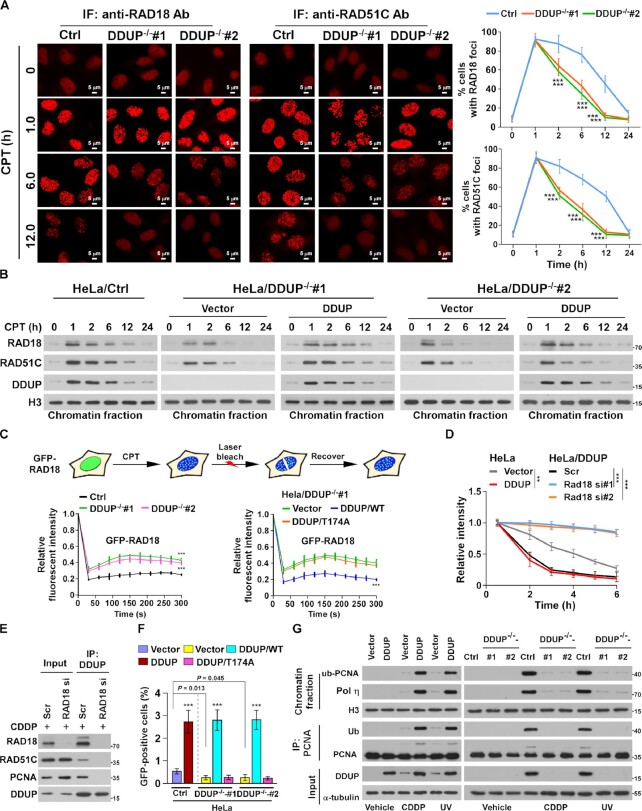
Figure 6.
DDUP enhances the retention of RAD18 at DNA damage sites. (A) Representative images (left) and time course (right) of the formation of CPT (10 μM)-induced RAD18 and RAD51C foci in control and DDUP-KO HeLa cells and allowed to recover for the indicated times. The RAD18- and RAD51C foci was examined every 10 min in the CPT-treated cells within the first 2 h. Cells containing more than 10 RAD18 and RAD51C foci per nucleus were scored. (B) Chromatin fraction and IB analysis of DNA-bound RAD18, RAD51C and DDUP in the indicated CPT (10 μM)-treated cells and allowed to recover for the indicated times. H3 served as a loading control. (C) Quantitative FRAP analysis of GFP-RAD18 in GFP-RAD18-transfected control and DDUP-KO HeLa cells (right), and in DDUP-KO HeLa cells co-transfected with GFP-RAD18 and vector, GFP-RAD18, and DDUP/WT, or GFP-RAD18 and DDUP/T174A, treated with CPT (10 μM) and allowed to recover for the indicated times. (D) Kinetics of γ-H2AX signals in the indicated cells in response to laser micro-irradiation and allowed to recover for the indicated times (n = 100). (E) IP assay analysis of the DDUP/RAD51C and DDUP/PCNA interaction in control and RAD18-silenced 293T cells treated with CDDP (5 μM, 1 h). (F) Homologous recombination repair assays performed in the indicated cells. (G) IP/IB analysis of the regulatory effect of DDUP dysregulation on PCNA monoubiquitination in the indicated cells treated with CDDP (5 μM, 1 h) or UV radiation (60 J/m2). H3 and α-tubulin served as loading control. Each error bar represents the mean ± SD of three independent experiments (*P< 0.05, **P< 0.01, ***P< 0.001).