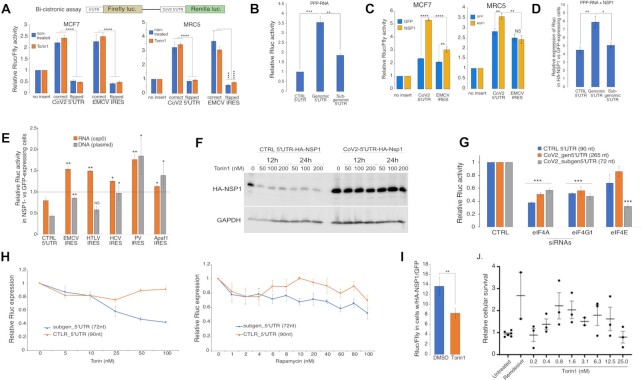
Figure 4.
The translational features of SARS-CoV-2 5′UTRs. (A) Upper panel: schematic representation of the bi-cistronic assay. MCF7 (left panel) or MRC5 (right panel) cells were transfected with bi-cistronic plasmids encoding for the SARS-CoV-2 genomic 5′UTR or EMCV-derived IRES element as a positive control. Both elements were introduced in their correct or flipped (i.e. anti-sense) configuration as a control for non-specific activity. Cells were grown for 24 h in standard medium (NT) or supplied with Torin1 (25 nM), after which Rluc and Ffly activities were assayed; n = 4, bars represent SE. (B) MRC5 cells were transfected with the indicated uncapped (PPP) in-vitro transcribed mRNAs bearing poly(A) tails. Rluc activity was tested 7 h later and is presented relative to the control plasmid-derived 5′UTR; n = 4, the bars represent SE. (C) MCF7 and MRC5 cells were transfected with i) the indicated bi-cistronic plasmids and ii) plasmid encoding either HA-NSP1 or eGFP. Luminescence was assayed 24 h later, n = 4, the bars represent SD. (D) MRC5 cells were transfected with plasmids encoding either HA-NSP1 or eGFP. After 24 h, uncapped RNAs with poly(A) tails encoding for indicated 5′UTRs and a downstream Rluc gene were transfected and Rluc activity was assayed 7 h later; n = 4, bars represent SE. (E) Grey columns: MRC5 cells were co-transfected with plasmids encoding i) either HA-NSP1 or eGFP and ii) indicated IRES sequences cloned before the Rluc reporter gene. Orange columns: MRC5 cells were transfected with plasmids encoding either HA-NSP1 or eGFP and, after 24 h, with capped mRNAs in vitro transcribed from the indicated IRES-containing plasmids; n = 2, bars represent SD. (F) HEK293 cells were transfected with plasmids encoding HA-NSP1 preceded by either control or viral 5′UTR and grown for 40 h post-transfection, out of which Torin1 was added during the last 12 or 24 h at the indicated concentrations. After harvest, total protein lysates were separated on 9% SDS-PAGE and probed with anti-HA and anti-GAPDH antibodies. (G) MRC5 cells were transfected with the indicated siRNAs, and re-transfected after 65 h with in-vitro transcribed mRNA reporters bearing both 5′caps and poly(A) tails. Rluc activity was tested 7 h after the second transfection and presented relatively to the cells expressing the control siRNA; n = 4, bars represent SE. (H) MRC5 cells were transfected with the indicated in-vitro transcribed mRNA reporters bearing both 5′caps and poly(A) tails. After 2 h, Torin1 (left panel) or Rapamycin (right panel) were added to yield the indicated concentrations and Rluc activity was tested after additional 5 h; n = 3, the bars represent SE. (I) MRC5 cells were transfected with the mix of plasmids encoding (i) either HA-NSP1 or eGFP, (ii) TSS-subgen_5′UTR-Rluc and, (iii) Firefly (Ffly). After 4 h of transfection, the medium was replaced and either DMSO or Torin1 (25 nM) were added. The cells were collected after 24 h, lysed and subjected to luminescence analysis. Rluc values were normalized against Ffly signal, n = 3, the bars represent SE. (J) Vero E6 cells were treated with the indicated final concentrations of Torin1 for 1 h, infected with SARS-CoV-2 at MOI = 0.01–0.015 and incubated for 3 days in the presence of Torin1. Following this time, the relative cytopathic effect of the virus was tested. As a positive control, cells were treated with Remdesivir (0.3 mM), for negative relative control cells were not treated prior to infection; n = 2 or 3, bars indicate SE.