Abstract
Objectives
Besides CD4 regulatory T-cells (Tregs), immunosuppressor FoxP3+ CD8 T-cells are emerging as an important subset of Tregs, which contribute to immune dysfunction and disease progression in HIV infection. However, FoxP3+ CD8 T-cell dynamics in acute HIV infection and following early antiretroviral therapy (ART) initiation remain understudied.
Methods
Subsets of FoxP3+ CD8 T-cells were characterized both prospectively and cross-sectionally in PBMCs from untreated acute (n=26) and chronic (n=10) HIV-infected individuals, early ART-treated in acute infection (n=10, median of ART initiation: 5.5 months post-infection), ART-treated in chronic infection (n=10), elite controllers (n=18), and HIV-uninfected controls (n=21).
Results
Acute and chronic infection were associated with increased total, effector memory, and terminally differentiated FoxP3+ CD8 T-cells, while early ART normalized only the frequencies of total FoxP3+ CD8 T-cells. We observed an increase in FoxP3+ CD8 T-cell immune activation (HLADR+/CD38+), senescence (CD57+/CD28-), and PD-1 expression during acute and chronic infection, which were not normalized by early ART. FoxP3+ CD8 T-cells in untreated participants expressed higher levels of immunosuppressive LAP(TGF-β1) and CD39 than uninfected controls, whereas early ART did not affect their expression. The expression of gut-homing markers CCR9 and Integrin-β7 by total FoxP3+ CD8 T-cells and CD39+ and LAP(TGF-β1)+ FoxP3+ CD8 T-cells increased in untreated individuals and remained higher than in uninfected controls despite early ART. Elite controllers share most of the FoxP3+ CD8 T-cell characteristics in uninfected individuals.
Conclusions
Although early ART normalized total FoxP3+ CD8 T-cells frequencies, it did not affect the persistent elevation of the gut-homing potential of CD39+ and LAP(TGF-β1)+ FoxP3+ CD8 T-cell, which may contribute to immune dysfunction.
Keywords: CD8 regulatory T cells (CD8 Tregs), acute HIV infection, early antiretroviral therapy (ART), FoxP3, TGF-β1, CD39
Introduction
Immunosuppressive CD8 T-cells are a heterogeneous group of suppressor T-cells with various origins, phenotypic characteristics, and suppressive mechanisms. Despite 50 years since their discovery (1), our understanding of the regulation and functions of these cells remains limited compared to their CD4 Treg counterparts, mainly due to the lack of specific characterization markers. However, various studies have revealed the undeniable role of immunosuppressive CD8 T-cells in cancer, autoimmune diseases, transplantation, and infectious diseases (2–6). Indeed, several CD8 T-cell populations with immunosuppressive capacity have been described, including those expressing FoxP3, the master transcription factor of Tregs, or other populations with immunosuppressive features in the absence of FoxP3 expression (7–9).
In physiological conditions, human CD4 T-cells usually express FoxP3 at higher levels than CD8 T-cells, and the frequencies of CD4+FoxP3+ T-cells are more elevated compared to FoxP3-expressing CD8 T-cells (around a 50-fold difference) (3, 10, 11). However, FoxP3 expression is crucial for the stability and functions of FoxP3+ CD8 T-cells (12, 13). Lim et al. demonstrated the presence and increased frequencies of FoxP3+ CD8 T-cells in the blood of HIV-infected individuals compared to uninfected controls (14, 15). They found a link between HIV disease progression and immune activation with the proportions of CD8+FoxP3+ T-cells while showing that FoxP3-expressing CD4 and CD8 T-cells in HIV-infected people are phenotypically distinct (15). SIV/HIV infections are associated with an increase in the frequencies of FoxP3+ CD8 T-cells that positively correlate with plasma viral load (VL), which negatively impact antiviral immune responses and contribute to HIV disease progression by inhibition of effector T-cell proliferation and cytokines secretion (15–18). Moreover, FoxP3+ CD8 T-cells induced after vaccination were critical in controlling SIV infection in Rhesus macaques (RM) by reducing CD4 T-cell activation and viral replication (19, 20). In a single report, higher FoxP3+ CD8 T-cell frequencies and absolute numbers were also observed in the blood of elite controller (EC) SIV-infected Indian RMs (18). However, the dynamics of FoxP3+ CD8 T-cells during acute HIV infection remain understudied.
Several subsets of FoxP3+ CD8 T-cells expressing highly immunosuppressive markers such as cytotoxic T lymphocyte antigen 4 (CTLA-4), programmed cell death protein-1 (PD-1), CD39, and transforming growth factor-beta 1 (TGF-β1) have been described (16, 17, 21–23). FoxP3+ CD8 T-cells express high levels of CTLA-4, which is needed for viral suppression in SIV-infected RMs (16, 17). Besides, CTLA-4 is required for FoxP3+ CD8 T-cells expansion, activation, and maintenance since the interaction CTLA-4/B7 promotes indoleamine 2,3-dioxygenase (IDO) expression by dendritic cells, further favoring the generation of CTLA-4+ FoxP3+ CD8 T-cells (22). PD-1 expression increases during SIV/HIV infections in correlation with immune activation, VL, and low CD4 T-cell count (24). PD-1/PD-1L contributes to the immunosuppressive functions of FoxP3+ CD8 T-cells (21). The ectonucleotidase CD39 hydrolyzes inflammatory ATP into ADP and AMP, followed by the generation of immunosuppressive adenosine in an orchestra with CD73 (25–27). The expression of CD39 by FoxP3+ CD8 T-cells is crucial for viral suppression in SIV-infected RMs (16). Fenoglio et al. found a positive correlation between the levels of CD39-expressing CD8 T-cells and VL, CD4 T-cell count and immune activation, suggesting their link with HIV disease progression (28). Furthermore, increased FoxP3+ CD8 T-cells in SIV infection correlated positively with TGF-β1 production (23). TGF-β1 limits effector T-cell proliferation while promoting the differentiation of both CD4 and FoxP3+ CD8 T-cells (12, 29, 30). TGF-β1 is first generated as a pro-TGF-β1, which is then cleaved to form a dimeric pro-peptide known as a latency-associated peptide (LAP), which binds non-covalently with mature TGF-β1 to prevent TGF-β1 binding to its receptor and subsequent activation (31). TGF-β1 production stimulates collagen-1 deposition and progressive lymphoid tissue fibrosis in SIV/HIV infections, starting during the acute infection (32, 33). Notably, TGF-β1-expressing CD8 T-cells are major contributors to fibrosis of lymph nodes and gut mucosal tissues during HIV infection regardless of the stage of the disease, antiretroviral therapy (ART) or disease outcome (34, 35).
Early ART initiation upon HIV exposure is highly recommended in clinical practice since it improves CD4 T-cell recovery and reduces VL and immune activation (36, 37). One study showed decreased FoxP3+ CD8 T-cell frequencies following early short-term ART in SIV-controllers RMs (38). Our team has recently reported that early ART initiation at four days post-infection can normalize CD39+ FoxP3+ CD8 T-cell frequencies in blood and mesenteric lymph nodes of progressor SIV-infected RMs (39). Moreover, we also recently reported an increase in total CD4 Tregs frequencies, which was normalized by early ART, while the frequencies of immunosuppressive CD4 Tregs-expressing CD39 and LAP(TGF-β1) with potential migration to the gut remained higher despite ART (40). However, to date, FoxP3+ CD8 T-cell dynamics during acute HIV infection and the impact of early ART initiation remain understudied.
Herein, we prospectively and cross-sectionally evaluated the dynamic of FoxP3+ CD8 T-cells during HIV infection and following early ART initiation in the acute phase. We found that despite decreasing frequencies of total FoxP3+ CD8 T-cells, early ART initiation failed to decrease the expansion of FoxP3+ CD8 T-cells with highly immunosuppressive functions and their potential migration to the gut, which may contribute to immune dysfunction and disease progression.
Material and methods
Study population
Frozen peripheral blood mononuclear cells (PBMCs) from HIV-infected individuals and uninfected controls were obtained from Montreal Primary and Slow Progressors HIV Infection cohorts and McGill University Health Centre. A total of 105 individuals were included in our study and our study has been carried out in both cross-sectional and longitudinal manners. In the cross-sectional analysis, 26 study participants had acute HIV infection, which was defined as being within 180 days after the estimated date of HIV infection (median (IQR) 90 (43–126) days). Individuals with chronic infection who had been infected for more than a year were left untreated (n=10) or given ART (n=11). HIV ECs (n=18) with CD4 count higher than 500 cells/ml in the absence of any treatment and undetectable plasma VL for at least 7 years, and 20 HIV-uninfected controls were also included in the cross-sectional analysis (Table 1). In addition, we followed longitudinally 20 acutely infected individuals overtime, ten of whom had started ART during the acute infection (median (IQR) 165 (97–212) days), and the other ten were left untreated (Table 1). Of note, our cross-sectional analysis did not include follow-up specimens from the longitudinal cohorts.
Table 1.
Clinical characteristics of study groups.
| Cross-sectional participants | Longitudinal participants | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Untreated | ART-Treated | |||||||||
| Characteristics | Non-infected (n=20) | Acute (n=26) | Chronic ART-(n=10) | Chronic ART+ (n=11) | EC (n=18) | p-values | Acute (n=10) | Chronic ART- (n=10) | Acute (n=10) | Chronic ART+ (n=10) |
|
Age, years
[median (IQR)] |
39
d
(30.75-47) |
39.5f,g
(32.75-43) |
32.5h,i
(26-39.75) |
51f,h
(41–60) |
49d,g,i
(32-55.5) |
0.0006 | 39.5 (35.50-43.25) |
39.5 (37.75-43) |
36 (29.75-46.50) |
36.5 (29-46.5) |
| Male sex, n (%) | 15
a
(75%) |
26a,g
(100%) |
10
i
(100%) |
11
j
(100%) |
10g,i,j
(55.6%) |
0.0002 | 10 (100%) |
10 (100%) |
10 (100%) |
10 (100%) |
|
CD4+ T-cells count, cells/µl
[median (IQR)] |
632
b
(463.5-775) |
460
g
(380–610) |
440b,i
(255–543) |
603 (400–847) |
730g,i
(638.5-900) |
0.001 | 515 (419-767.5) |
595 (287.5-813.8) |
450
l
(272.5-561.3) |
521
l
(377.5-795) |
|
CD8+ T-cells count, cells/µl
[median (IQR)] |
197a,b,c,d
(153-428.5) |
996
a
(640–1630) |
750
b
(629-1133) |
743
c
(433.3-1192) |
739
d
(604-1040) |
0.0002 | 830
k
(615-1170) |
953
k
(705-1915) |
1019 (580-1708) |
655 (531-1081) |
|
CD4/CD8 ratio
[median (IQR)] |
2.82a,b,c,d
(1.41-4.19) |
0.46a,f,g
(0.21-1.14) |
0.48b,h,i
(0.40-0.61) |
0.87c,f,h
(0.60-1.81) |
0.95d,g,i
(0.80-1.43) |
< 0.0001 | 0.56
k
(0.40-1.35) |
0.50
k
(0.32-0.85) |
0.40
l
(0.19-0.81) |
0.69
l
(0.40-1.24) |
|
Nadir CD4+ T-cells count, cells/µl
[median (IQR)] |
NA | 330 (257.8-500) |
310 (245-423.5) |
334 (297.8-533.5) |
551.5 (301.5-624.8) |
0.30 | 365 (297.5-525) |
NA | 258.5 (207.5-530) |
NA |
|
Viral load, log10 copies/mL
[median (IQR)] |
NA | 4.36f,g
(3.82-5.50) |
4.56h,i
(3.74-3.98) |
1.60f,h
(1.60-1.60) |
1.65g,i
(1.60-1.69) |
< 0.0001 | 4.07
k
(3.54-4.37) |
4.60
k
(4.01-5.20) |
4.40
l
(3.92-5.77) |
1.70
l
(1.67-1.70) |
|
Duration of infection, years
[median (IQR)] |
NA | 0.25e,f,g
(0.12-0.35) |
2.55e,h,i
(1.54-4.26) |
12.40f,h
(4.99-19.33) |
15.3g,i
(7.87-21) |
< 0.0001 | 0.22
k
(0.11-0.36) |
2.19
k
(2.12-2.39) |
0.28
l
(0.13-0.39) |
2.27
l
(2.00-2.65) |
| Time of ART initiation years post-infection [median (IQR)] | NA | NA | NA | 1.11 (0.49-2.02) |
NA | NA | NA | NA | 0.46 (0.27-0.59) |
|
|
Duration of ART, years
[median (IQR)] |
NA | NA | NA | 14.58 (3.56-20.73) |
NA | NA | NA | NA | 1.72 (1.43-199) |
|
Results are shown as median and interquartile range (IQR).
NA, not applicable; EC, Elite controllers.
p-values come from comparing the six groups using the Kruskal-Wallis test. Significant differences (p < 0.05) following Mann–Whitney U test or Fisher’s test are mentioned as follow:
: Non-infected vs Acute,
: Non-infected vs Chronic (ART-),
: Non infected vs Chronic (ART+),
: Non-infected vs EC
: Acute vs Chronic (ART-),
: Acute vs Chronic (ART+),
: Acute vs EC,
: Chronic (ART-) vs Chronic (ART+),
: Chronic (ART-) vs EC,
: Chronic (ART+) vs EC, Significant differences (p < 0.05) following Wilcoxon signed-rank test are mentioned as follow:
: Acute vs Chronic (ART-),
: Acute vs Chronic (ART+)
Ethical considerations
The Ethical Review Board of the Université du Québec à Montréal (UQAM) gave their approval to this study (#2014-452), which followed the Declaration of Helsinki. All study participants signed a written informed consent form before blood collection.
Flow cytometry analysis
Multiparameter flow cytometry was performed on thawed PBMCs. For immunological staining, the optimal concentrations of fluorochrome-conjugated antibodies were used in 3 independent panels of 14 colors each, as shown in Supplementary Table 1 . The LIVE/DEAD Fixable Aqua Dead Cell Stain Kit (Invitrogen, Oregon, USA) was used to eliminate dead cells from the analysis. After extracellular staining, cells were permeabilized with the Transcription Factor Buffer Set (BD Bioscience, New Jersey, USA) and further stained intracellularly for FoxP3 and CTLA-4. The data was collected using a three-laser BD LSR Fortessa X-20 cytometer, and the results were analyzed using FlowJo V10.8.1 (Oregon, USA).
Statistical analysis
GraphPad Prism V6.01 (California, USA) was used for statistical analysis. The results are shown as medians with an interquartile range (IQR) throughout the text. The distribution of variables was initially determined by the Kolmogorov–Smirnov test. The Kruskal–Wallis test was then used to evaluate any statistically significant differences between the five study groups. Nonparametric Mann-Whitney was used for unpaired variables, while the Wilcoxon rank tests were used for paired analysis. The correlation between variables was determined using the Spearman correlation coefficient test. Only statistical significances (p<0.05) are presented in the figures (*, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001).
Results
Characteristics of the study populations
ECs and chronically infected participants on ART were older than individuals in other study groups and had been infected for a longer period of time (Table 1). In chronic infection, ART restored CD4 T-cell count but was unable to normalize both CD8 T-cell levels and CD4/CD8 ratio compared to the uninfected group. ECs had similar CD4 T-cell count to those of uninfected individuals but had higher CD8 T-cell count and lower CD4/CD8 ratio. Chronically infected individuals on ART in the cross-sectional study were significantly older (median age: 51 versus 36.5 years, Mann-Whitney p= 0.04), had a longer duration of HIV infection (median: 12.7 versus 2.27 years, p< 0.0001), and had been longer on ART (median: 14.58 versus 1.72 years, Mann-Whitney p= 0.0002) than ART-treated chronically infected individuals in the longitudinal group. Furthermore, ART was initiated earlier in the longitudinal group (median years of ART initiation post-infection: 1.11 versus 0.46 years, Mann-Whitney p= 0.05). We thus evaluated the effect of early ART initiation on FoxP3+ CD8 T-cell in the longitudinal analysis. Early ART initiation improved CD4 T-cell count (Wilcoxon p= 0.04) and CD4/CD8 ratio (Wilcoxon p=0.002). Moreover, there was no significant difference in clinical characteristics between two (n=10) acute individuals in untreated and ART-treated longitudinal specimens.
HIV infection is associated with a rapid expansion of total FoxP3+ CD8 T-cells, which was normalized by early ART initiation
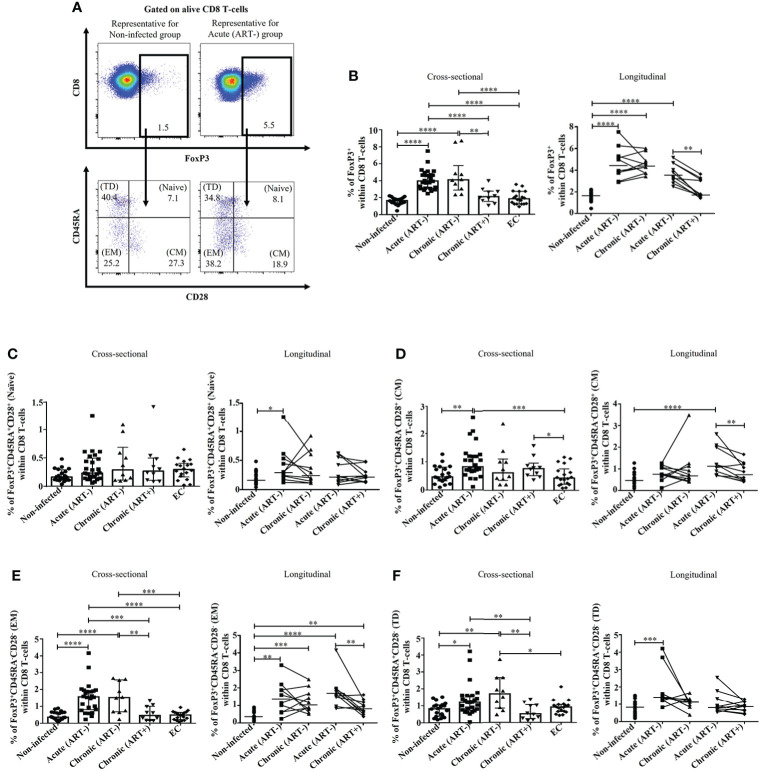
Untreated HIV infection increased FoxP3+CD8+ T-cells frequencies beginning in the acute phase compared to uninfected individuals (p< 0.0001 in both cross-sectional and longitudinal analysis), which was normalized by early ART initiation (Figures 1A, B). The frequency of total FoxP3+ CD8 T-cells was inversely correlated with CD4 T-cell count and CD4/CD8 ratio and positively with plasma viral load and both CD4 and CD8 immune activation (Table 2). Total FoxP3+ CD8 T-cells in ECs were significantly lower than untreated HIV-infected individuals (p< 0.0001 for both acute and ART- chronic) like uninfected controls (Figures 1A, B).
Figure 1.
Effect of early ART initiation on total FoxP3+ CD8 T-cells frequencies and memory subsets. (A) Gating strategies used in flow cytometry to define total FoxP3 + CD8 T-cells and FoxP3 + CD8 T-cells memory subsets within CD8 T-cells. (B) Percentages of total FoxP3 + CD8 T-cells. Frequencies of (C) naïve (CD45RA+CD28+), (D) central memory (CM, CD45RA-CD28+) (E) effector memory (EM, CD45RA-CD28-), and (F) terminally differentiated (TD, CD45RA+CD28-) FoxP3 + CD8 T-cells subsets. Statistical significance is indicated in the figures as follow: *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001. Differences among five study groups was determined by nonparametric Mann-Whitney rank test for unpaired variables, while the Wilcoxon rank tests were used for paired variables in the longitudinal study. Sample size in cross-sectional analysis: non-infected n=20, Acute n=26, Chronic ART- n=10, Chronic ART+ n=11, EC n=18. Sample size in longitudinal analysis: non-infected n=20, ART- n=10, ART+ n=10.
Table 2.
Correlation between clinical and immunological parameters and CD4 T-cell count, CD4/CD8 ratio, CD4 activation (CD4+HLA-DR+CD38+), CD8 activation (CD8+HLA-DR+CD38+), plasma viral load (log10/ml), and duration of ART (years).
| CD4 T-cell count (cells/µl) | CD4/CD8 ratio | CD4 activation (HLA-DR+CD38+) | CD8 activation (HLA-DR+CD38+) | Plasma viral load (log10/ml) | Duration of treatment (years) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| r | p | r | p | r | p | r | p | r | p | r | p | |
| CD8+FoxP3+ | -0.424 | 0.0001 | -0.4236 | 0.0001 | 0.6332 | < 0.0001 | 0.678 | < 0.0001 | 0.6005 | < 0.0001 | -0.1089 | 0.68 |
| CD8+FoxP3+CD45RA+CD28+ (Naïve) | 0.07659 | 0.51 | 0.1419 | 0.22 | 0.1853 | 0.09 | 0.1797 | 0.1 | -0.06796 | 0.59 | -0.3841 | 0.14 |
|
CD8+FoxP3+CD45RA-CD28+
(CM) |
-0.1267 | 0.27 | -0.06221 | 0.59 | 0.2651 | 0.01 | 0.4002 | 0.0002 | 0.2394 | 0.06 | -0.2987 | 0.25 |
|
CD8+FoxP3+CD45RA-CD28-
(EM) |
-0.4184 | 0.0002 | -0.5109 | < 0.0001 | 0.4998 | < 0.0001 | 0.5 | < 0.0001 | 0.597 | < 0.0001 | 0.03091 | 0.91 |
|
CD8+FoxP3+CD45RA+CD28-
(TD) |
-0.09472 | 0.41 | -0.1506 | 0.19 | 0.3426 | 0.001 | 0.171 | 0.11 | 0.233 | 0.06 | -0.2471 | 0.35 |
| CD8+FoxP3+CD38+HLA-DR+ | -0.4041 | 0.0003 | -0.514 | < 0.0001 | 0.7546 | < 0.0001 | 0.7502 | < 0.0001 | 0.6481 | < 0.0001 | -0.4812 | 0.05 |
| CD8+FoxP3+CD57+CD28- (Senescent) | -0.3267 | 0.004 | -0.5553 | < 0.001 | 0.5502 | < 0.0001 | 0.4332 | < 0.0001 | 0.452 | 0.0002 | -0.5 | 0.05 |
| CD8+FoxP3+PD-1+ | -0.4425 | < 0.0001 | -0.4824 | < 0.0001 | 0.6112 | < 0.0001 | 0.6657 | < 0.0001 | 0.6954 | < 0.0001 | -0.06034 | 0.81 |
| CD8+FoxP3+CTLA-4+ | -0.1377 | 0.23 | -0.2272 | 0.04 | 0.3825 | 0.003 | 0.5703 | < 0.0001 | 0.08438 | 0.51 | 0.2471 | 0.35 |
| CD8+FoxP3+CD39+ | -0.1306 | 0.26 | -0.11 | 0.34 | 0.4296 | < 0.0001 | 0.5198 | < 0.0001 | 0.0468 | 0.71 | -0.5353 | 0.03 |
| CD8+FoxP3+LAP(TGF-β1)+ | -0.125 | 0.28 | -0.2067 | 0.07 | 0.287 | 0.008 | 0.4102 | 0.0001 | -0.1189 | 0.35 | 0.3353 | 0.2 |
| CD8+FoxP3+CD39+LAP(TGF-β1)+ | -0.01223 | 0.91 | 0.03104 | 0.79 | 0.2494 | 0.02 | 0.3246 | 0.002 | -0.1501 | 0.24 | -0.25 | 0.34 |
| CD8+FoxP3+CCR4+ | -0.2196 | 0.05 | -0.1122 | 0.33 | 0.2996 | 0.005 | 0.4384 | < 0.0001 | 0.119 | 0.3569 | 0.02504 | 0.92 |
| CD8+FoxP3+CCR5+ | 0.000274 | 0.99 | 0.02539 | 0.82 | 0.2145 | 0.05 | 0.3031 | 0.005 | 0.2602 | 0.04 | -0.1441 | 0.59 |
| CD8+FoxP3+CCR6+ | -0.3175 | 0.005 | -0.2807 | 0.01 | 0.5175 | < 0.0001 | 0.4891 | < 0.0001 | 0.4594 | 0.0002 | 0.07959 | 0.76 |
| CD8+FoxP3+CXCR3+ | -0.3366 | 0.003 | -0.4452 | <0.0001 | 0.6352 | < 0.0001 | 0.7059 | < 0.0001 | 0.54 | < 0.0001 | -0.02647 | 0.92 |
| CD8+FoxP3+CCR9+ | -0.2133 | 0.06 | -0.2591 | 0.02 | 0.5683 | < 0.0001 | 0.6669 | < 0.0001 | 0.3099 | 0.01 | -0.7235 | 0.002 |
| CD8+FoxP3+Integrin β7+ | -0.3791 | 0.0007 | -0.4641 | < 0.0001 | 0.5138 | < 0.0001 | 0.6631 | < 0.0001 | 0.4482 | 0.0003 | -0.2931 | 0.26 |
| CD8+FoxP3+CCR9+CD39+ | -0.1289 | 0.26 | -0.1451 | 0.21 | 0.4407 | < 0.0001 | 0.5628 | < 0.0001 | 0.139 | 0.28 | -0.5284 | 0.03 |
| CD8+FoxP3+CCR9+LAP(TGF-β1)+ | -0.1275 | 0.27 | -0.1418 | 0.22 | 0.4638 | < 0.0001 | 0.5614 | < 0.0001 | 0.1825 | 0.15 | -0.571 | 0.02 |
| CD8+FoxP3+Integrin β7+CD39+ | -0.1667 | 0.15 | -0.1665 | 0.15 | 0.3779 | 0.0004 | 0.5338 | < 0.0001 | 0.2866 | 0.02 | -0.546 | 0.03 |
| CD8+FoxP3+Integrin β7+LAP(TGF-β1)+ | -0.1987 | 0.08 | -0.2806 | 0.01 | 0.3916 | 0.0002 | 0.5249 | < 0.0001 | 0.1612 | 0.21 | 0.3265 | 0.21 |
| CD28-PD-1+ CD8 T-cells | -0.1828 | 0.11 | -0.2973 | 0.009 | 0.2920 | 0.007 | 0.4417 | <0.0001 | -0.0969 | 0.45 | -0.09706 | 0.72 |
| CD28-CD39+ CD8 T-cells | -0.03237 | 0.78 | -0.2038 | 0.07 | 0.2188 | 0.04 | 0.2443 | 0.02 | -0.0443 | 0.73 | -0.5107 | 0.04 |
p-values come from the comparison of clinical and immunological parameters with CD4/CD8 ratio, CD4 activation (CD4+HLA-DR+CD38+), CD8 activation (CD8+HLA-DR+CD38+), plasma viral load (log10/ml), and duration of ART (years) by using the Spearman correlation coefficient test.
Significant differences (p < 0.05) are highlighted in Bold.
We observed a marked heterogeneity in FoxP3+ CD8 T-cell subsets based on CD28 and CD45RA expression (27, 41) in untreated HIV-infected individuals compared to ART-treated and uninfected controls (Figure 1). The frequencies of naïve (CD45RA+CD28+) FoxP3+ CD8 T-cells remained unchanged in all study groups in the cross-sectional analysis, and only a significant increase was observed in acutely infected individuals in the longitudinal study (p= 0.01) (Figure 1C). In addition, acute HIV infection compared to uninfected controls, was linked to increased frequencies of central memory (CM, CD45RA-CD28+) (p= 0.001), effector memory (EM, CD45RA-CD28-) (p< 0.0001), and terminally differentiated (TD, CD45RA+CD28-) FoxP3+ CD8 T-cells (p= 0.01) (Figures 1D-F). Early ART initiation did not affect naïve and TD FoxP3+ CD8 T-cells but decreased the frequencies of both CM and EM FoxP3+ CD8 T-cells. Despite early ART, the frequencies of EM FoxP3+ CD8 T-cells remained higher than in uninfected controls (p= 0.001) (Figure 1E). Frequencies of CM FoxP3+ CD8 T-cells positively correlated with CD4 and CD8 immune activation (Table 2). Both CD4 and CD8 immune activation positively correlated with frequencies of EM and TD FoxP3+ CD8 T-cells, whereas only EM FoxP3+ CD8 T-cells were positively associated with VL and inversely with CD4 T-cell count and CD4/CD8 ratio (Table 2). ECs showed lower CM FoxP3+ CD8 T-cells frequencies compared to chronic (ART+) and acute (ART-), lower EM FoxP3+ CD8 T-cells than chronic (ART-) and acute (ART-), and lower TD FoxP3+ CD8 T-cells compared to chronic (ART-) (Figures 1C, E, F). Overall, our results showed an increased differentiation of FoxP3+ CD8 T-cells in acute infection that, except for EM CD8 FoxP3+ CD8 T-cells, was normalized by early ART.
Early ART initiation decreased but not normalized immune activation and senescence of FoxP3+ CD8 T-cells
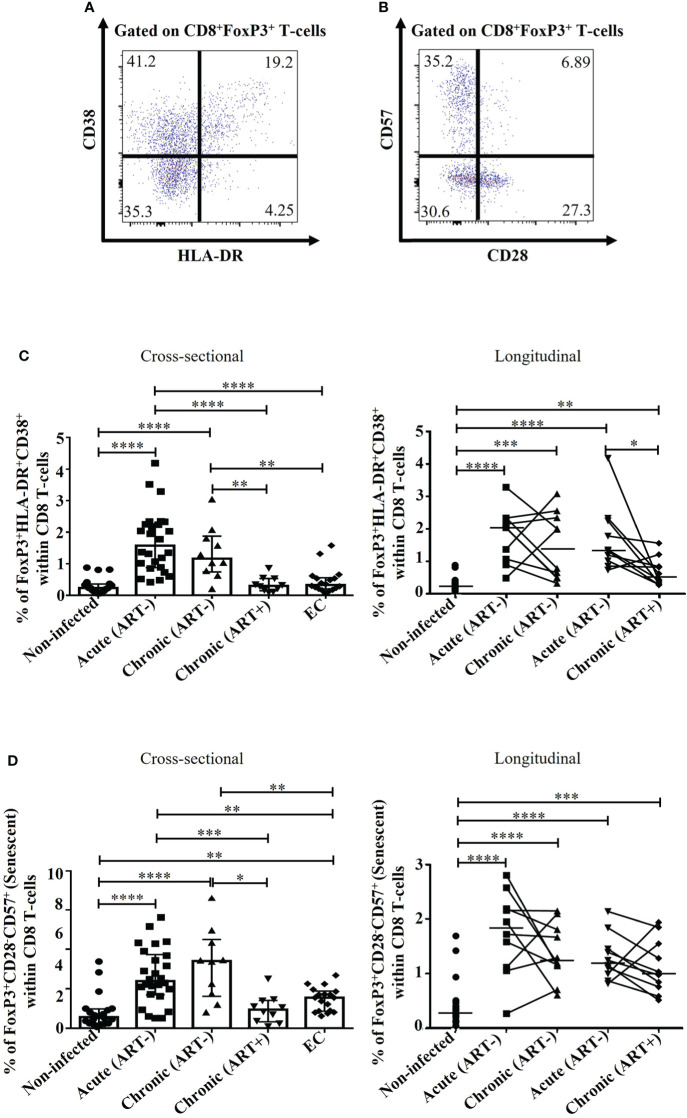
HIV infection was associated with increased frequencies of activated CD38+/HLA-DR+ FoxP3+ CD8 T-cell compared to uninfected individuals (p< 0.0001 for all comparisons in both acute (ART-) and chronic (ART-); Figures 2A, C). Although early ART initiation decreased FoxP3+ CD8 T-cell activation (CD38+HLA-DR+), it could not normalize their levels (Figure 2C). ECs had similar FoxP3+ CD8 T-cell activation than uninfected controls and showed significantly lower frequencies of activated FoxP3+ CD8 T-cell compared to acute and chronic ART- individuals (Figure 2C). HIV infection was associated with increased senescent (CD28-CD57+) FoxP3+ CD8 T-cells (p< 0.0001 in both cross-sectional and longitudinal analysis), while early ART initiation failed to normalize their frequencies (p=0.0001) (Figures 2B, D). Interestingly, ECs had lower frequencies of senescent FoxP3+ CD8 T-cells compared to ART- HIV-infected individuals, but these frequencies were higher than uninfected controls (p= 0.001) (Figure 2D). The frequency of activated and senescent FoxP3+ CD8 T-cells was inversely correlated with CD4 T-cell count and CD4/CD8 ratio and positively with plasma viral load and CD4 and CD8 immune activation (Table 2). Altogether, our results indicate that early ART initiation failed to normalize FoxP3+ CD8 T-cells immune activation and senescence.
Figure 2.
Effect of early ART initiation on FoxP3+ CD8 T-cell immune activation and senescence. (A) Gating strategies used in flow cytometry to define activated FoxP3 + CD8 T-cells (CD8+FoxP3+HLA-DR+CD38+). (B) Gating strategies used in flow cytometry to define immunosenescent FoxP3 + CD8 T-cells (CD8+FoxP3+CD28-CD57+). Frequencies of CD8+FoxP3+HLA-DR+CD38+ (C), and CD8+FoxP3+CD28-CD57+ (D) within CD8 T-cells. Statistical significance is indicated in the figures as follow: *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001. Differences among five study groups was determined by nonparametric Mann-Whitney rank test for unpaired variables, while the Wilcoxon rank tests were used for paired variables in the longitudinal study. Sample size in cross-sectional analysis: non-infected n=20, Acute n=26, Chronic ART- n=10, Chronic ART+ n=11, EC n=18. Sample size in longitudinal analysis: non-infected n=20, ART- n=10, ART+ n=10.
Impact of early ART initiation on immunosuppressive subsets of FoxP3+ CD8 T-cells
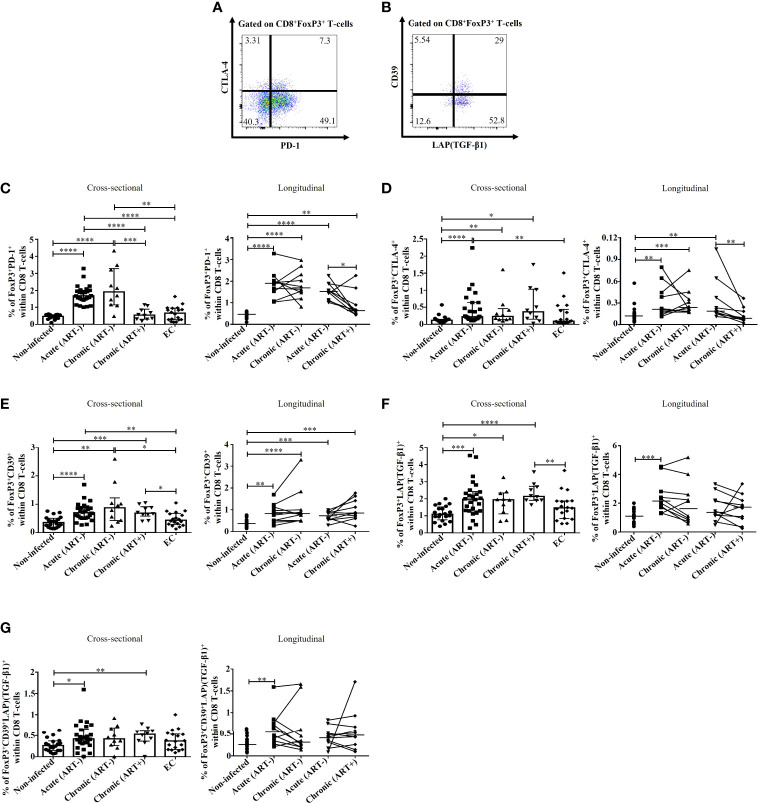
As previously mentioned, FoxP3+ CD8 T-cells include various subsets based on the expression of PD-1 (21), CTLA-4 (16, 17, 22), CD39 (16), and TGF-β1 (12, 23, 30), which are needed for their survival and to exert immunosuppressive functions (Figures 3A, B ). HIV infection was linked to an increase in the frequencies of PD-1+ FoxP3+ CD8 T-cells (p< 0.0001 for both acute and chronic ART-) and CTLA-4+ FoxP3+ CD8 T-cells (p< 0.0001, p= 0.003 for acute and chronic ART-, respectively) compared to uninfected individuals (Figures 3C, D). Early ART initiation normalized CTLA-4+ FoxP3+ CD8 T-cells but not PD-1+ FoxP3+ CD8 T-cells (Figures 3C, D). These two populations were inversely correlated with CD4/CD8 ratio and positively with CD4 and CD8 immune activation (Table 2). In addition, only PD-1+ FoxP3+ CD8 T-cells negatively correlated with CD4 T-cell count and positively with VL (Table 2). ECs presented lower frequencies of PD-1+ (p< 0.0001 for both acute and chronic ART-) and CTLA-4+ (p= 0.009 for acute) FoxP3+ CD8 T-cells compared with ART- HIV-infected individuals and similar to uninfected controls (Figures 3C, D). HIV infection was also associated with increased frequencies of CD39+ FoxP3+ CD8 T-cells in both acute and chronic phases, and ART had no impact on their frequencies, while ECs represent similar frequencies of CD39+ FoxP3+ CD8 T-cells than uninfected controls (Figure 3E). CD39+ FoxP3+ CD8 T-cells frequencies correlated positively with CD4 and CD8 activation, whereas ART duration negatively correlated with this population (Table 2).
Figure 3.
Effect of early ART initiation on FoxP3+ CD8 T-cells subsets with known immunosuppressive functions. Gating strategies used in flow cytometry to define FoxP3 + CD8 T-cells expressing PD-1/CTLA-4 (A) and CD39/LAP(TGF-β1) (B). Frequencies of CD8+FoxP3+PD-1+ (C), CD8+FoxP3+CTLA-4+ (D), CD8+FoxP3+CD39+ (E), CD8+FoxP3+LAP(TGF-β1)+ (F), and CD8+FoxP3+CD39+LAP(TGF-β1)+ (G) within CD8 T-cells. Statistical significance is indicated in the figures as follow: *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001. Differences among five study groups was determined by nonparametric Mann-Whitney rank test for unpaired variables, while the Wilcoxon rank tests were used for paired variables in the longitudinal study. Sample size in cross-sectional analysis: non-infected n=20, Acute n=26, Chronic ART- n=10, Chronic ART+ n=11, EC n=18. Sample size in longitudinal analysis: non-infected n=20, ART- n=10, ART+ n=10.
LAP(TGF-β1)+ and CD39+LAP(TGF-β1)+ FoxP3+ CD8 T-cell frequencies were increased in HIV acute infection compared to uninfected controls and positively correlated with CD4 and CD8 T-cell immune activation (Figures 3F, G; Table 2). Early ART initiation, but not later ART in cross-sectional analysis, inhibited their expansion (Figures 3F, G). Overall, our data demonstrated that early ART initiation reduced the frequencies of immunosuppressive CTLA-4+ FoxP3+ CD8 T-cells but was unable to normalize the frequencies of other immunosuppressive FoxP3+ CD8 T-cell subsets.
Impact of HIV infection and early ART on migration potential of FoxP3+ CD8 T-cells
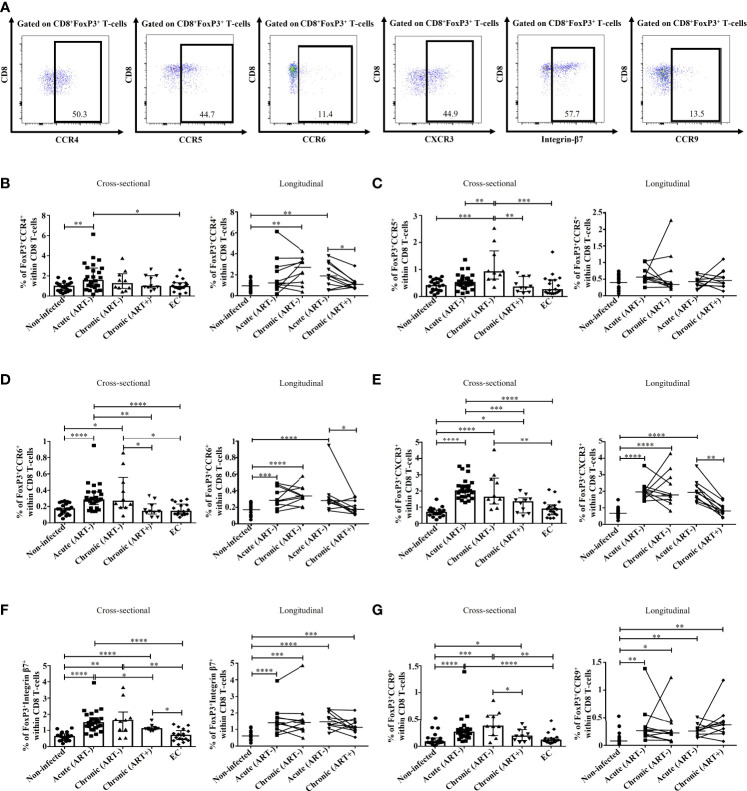
We then evaluated the potential migration of FoxP3+ CD8 T-cells by characterizing the expression of chemokine receptors (Figure 4A). CCR4 binds to chemokine ligands CCL17 and CCL22 and is mainly expressed by T-cells. The expression of CCR4 has been linked to migration to the skin, heart, lung, and lymph nodes (42–45). CCR4+ FoxP3+ CD8 T-cell frequencies were higher in acute HIV infection compared to uninfected controls and ECs (p= 0.006 and p= 0.02, respectively), which was normalized by early ART initiation (Figure 4B). The frequencies of this population positively correlated with CD4 and CD8 immune activation (Table 2). CCR5 is a G-coupled receptor that binds to CCL3, CLL4, and CCL5 linked to cell migration to the brain, inflamed tissues and gut and is suggested to play an essential role in CD8 T-cells differentiation and activation (46–49). Significant increases in CCR5+ FoxP3+ CD8 T-cell frequencies were found only in the chronic ART- group, while ART+ individuals represented similar levels of CCR5+ FoxP3+ CD8 T-cells than uninfected controls and ECs (Figure 4C). CXCR3 is an IFN-inducible chemokine receptor that binds to chemokines CXCL4, CXCL9, CXCL10, and CXCL11, which directs the migration towards inflamed sites (50–52). We also assessed the expression of CCR6 (53, 54), CCR9 (55–57), and Integrin-β7 (53, 57), which direct T-cells recruitment towards the gut through the binding of CCL20, CCL25, and Mucosal vascular-Addressin Cell-Adhesin Molecule 1, respectively. Frequencies of CCR6+ and CXCR3+ FoxP3+ CD8 T-cells were increased in untreated acute and chronic ART- HIV infection (Figures 4D, E). Similarly, FoxP3+ CD8 T-cells expressing gut homing markers CCR9 and Integrin-β7 were also rapidly increased in acute and chronic ART- infection. However, in contrast to CCR6 and CXCR3, early ART initiation was unable to normalize the levels of CCR9+ and Integrin-β7+ FoxP3+ CD8 T-cells (Figures 4F, G ). ECs have a similar expression of these chemokine receptors than uninfected controls (Figures 4F, G ). The frequencies of CCR6+, CXCR3+, CCR9+, and Integrin-β7+ FoxP3+ CD8 T-cells were negatively correlated with CD4 T-cell count and CD4/CD8 ratio, while positively correlated with T-cell immune activation and plasma VL (Table 2). Moreover, only CCR9+ FoxP3+ CD8 T-cells inversely correlated with the duration of ART (Table 2).
Figure 4.
Effect of early ART initiation on FoxP3+ CD8 T-cells potential migration. (A) Gating strategies used in flow cytometry to define CD8+FoxP3+ T-cells -expressing CCR4, CCR5, CCR6, CXCR3, Integrin-β7 and CCR9. Frequencies of CD8+FoxP3+CCR4+ (B), CD8+FoxP3+CCR5+ (C), CD8+FoxP3+CCR6+ (D), CD8+FoxP3+CXCR3+ (E), CD8+FoxP3+Integrin-β7+ (F), and CD8+FoxP3+CCR9+ (G) within CD8 T-cells. Statistical significance is indicated in the figures as follow: *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001. Differences among five study groups was determined by nonparametric Mann-Whitney rank test for unpaired variables, while the Wilcoxon rank tests were used for paired variables in the longitudinal study. Sample size in cross-sectional analysis: non-infected n=20, Acute n=26, Chronic ART- n=10, Chronic ART+ n=11, EC n=18. Sample size in longitudinal analysis: non-infected n=20, ART- n=10, ART+ n=10.
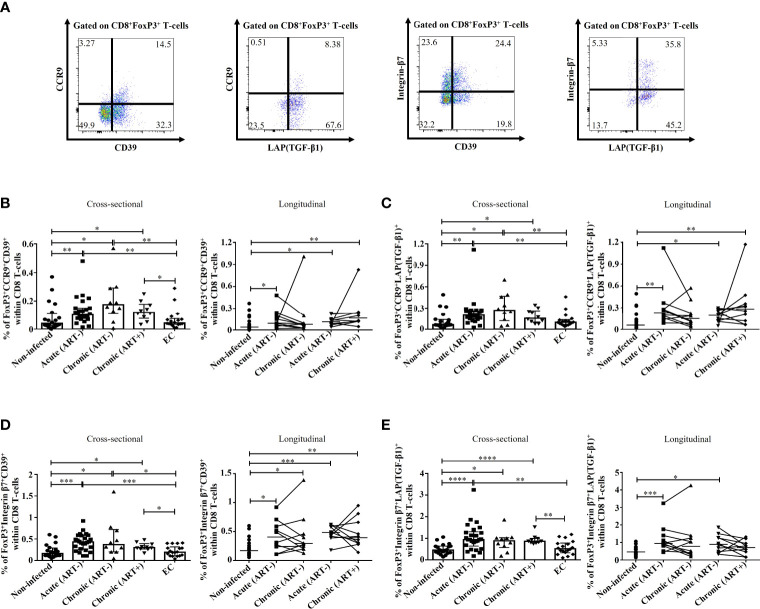
We also assessed the potential migration of immunosuppressive CD39+ and LAP(TGF-β1)+ FoxP3+ CD8 T-cells towards the gut by their expression of CCR9 and Integrin-β7. Here again, we observed that untreated HIV infection was associated with increases in CD39+ and LAP(TGF-β1)+ FoxP3+ CD8 T-cells expressing CCR9 and Integrin-β7 compared to ECs and uninfected controls (Figure 5). Importantly, early ART initiation failed to normalize the gut migration potential of these subsets expect for Integrin-β7+LAP(TGF-β1)+ FoxP3+ CD8 T-cells. The frequencies of CD39+ and LAP(TGF-β1)+ FoxP3+ CD8 T-cells expressing CCR9 and Integrin-β7 were all positively correlated with T-cell immune activation, and, excepting Integrin β7+LAP(TGF-β1)+, they negatively correlated with longer duration of ART (Table 2). Furthermore, we found a positive correlation between VL and Integrin β7+CD39+ FoxP3+ CD8 T-cells frequencies, whereas Integrin β7+LAP(TGF-β1)+ FoxP3+ CD8 T-cells were negatively correlated with CD4/CD8 ratio (Table 2). Altogether, our results showed that during HIV infection and despite early ART initiation, immunosuppressive CD39+ and LAP(TGF-β1)+ FoxP3+ CD8 T-cells maintained their capacity to migrate to the gut, which, in turn, could contribute to gut mucosal immune dysfunction and tissue fibrosis.
Figure 5.
Effect of early ART initiation on the migratory potential towards the gut of FoxP3+ CD8 T-cells subsets with known immunosuppressive functions. (A) Gating strategies used in flow cytometry to define CCR9+CD39+, CCR9+LAP(TGF-β1)+, Integrin-β7+CD39+, and Integrin-β7+LAP(TGF-β1)+ CD8+FoxP3+ T-cells. Frequencies of CD8+FoxP3+CCR9+CD39+ (B), CD8+FoxP3+CCR9+LAP(TGF-β1)+ (C), CD8+FoxP3+Integrin-β7+CD39+ (D), and CD8+FoxP3+Integrin-β7+LAP(TGF-β1)+ (E) within CD8 T-cells. Statistical significance is indicated in the figures as follow: *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001. Differences among five study groups was determined by nonparametric Mann-Whitney rank test for unpaired variables, while the Wilcoxon rank tests were used for paired variables in the longitudinal study. Sample size in cross-sectional analysis: non-infected n=20, Acute n=26, Chronic ART- n=10, Chronic ART+ n=11, EC n=18. Sample size in longitudinal analysis: non-infected n=20, ART- n=10, ART+ n=10.
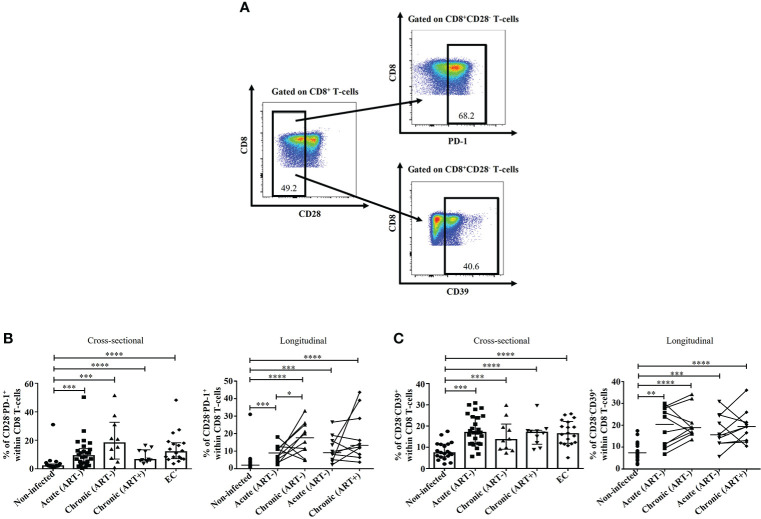
Early ART was unable to normalize CD28-PD-1+ and CD28-CD39+ CD8 T-cell subsets
In addition to classical FoxP3+ CD8 T-cells, other CD8 T-cell subsets have also been described as immunosuppressive regardless of their FoxP3 expression, including CD8+CD28-PD-1+ and CD8+CD28-CD39+ CD8 T-cells (7, 8, 28, 58). Herein, we observed a rapid expansion of both CD28-PD-1+ and CD28-CD39+ CD8 T-cell subsets in untreated HIV infection compared to ECs and uninfected controls (Figure 6). Importantly, their frequencies were not affected by early ART initiation. Both populations correlated positively with CD4 and CD8 immune activation, while only CD28-PD-1+ negatively correlated with CD4/CD8 ratio, and only CD28-CD39+ negatively correlated with the duration of ART (Table 2).
Figure 6.
Effect of early ART initiation on CD8+CD28-PD-1+ and CD8+CD28-CD39+ T-cells. Gating strategies used in flow cytometry to define CD8+CD28-PD-1+ and CD8+CD28-CD39+ (A). Frequencies of CD8+CD28-PD-1+ (B) and CD8+CD28-CD39+ (C) within CD8 T-cells. Statistical significance is indicated in the figures as follow: *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001. Differences among five study groups was determined by nonparametric Mann-Whitney rank test for unpaired variables, while the Wilcoxon rank tests were used for paired variables in the longitudinal study. Sample size in cross-sectional analysis: non-infected n=20, Acute n=26, Chronic ART- n=10, Chronic ART+ n=11, EC n=18. Sample size in longitudinal analysis: non-infected n=20, ART- n=10, ART+ n=10.
Discussion
The immune responses exerted by effector CD8 T-cells are crucial for controlling SIV/HIV infections (59, 60). In contrast, immunosuppressive functions of FoxP3+ CD8 T-cells are primarily detrimental since higher frequencies of these cells have been associated with immune dysfunction, viral persistence and HIV disease progression (15–17). We recently showed that early ART initiation in HIV-infected individuals was unable to reduce CD39+ and LAP(TGF-β1)+ CD4 Tregs and their potential migration to the gut (40). Herein, in the same study cohort, we showed that acute HIV infection increased the frequencies of FoxP3+ CD8 T-cells, which were normalized by early ART initiation. Importantly, although we observed an overall increase in FoxP3 expression on CD8 T-cells in untreated HIV infection that could affect the relative proportions of FoxP3+ subsets reported in our study, we also observed clear differences between the expression of various markers on FoxP3+ CD8 T-cells versus total CD8 T-cells which suggest the particular dynamics of FoxP3+ CD8 T-cells during HIV infection (Supplementary Table 2). In contrast, early treatment was unable to normalize FoxP3+ CD8 T-cell activation and senescence, as well as the gut migratory potential of CD39+ and LAP(TGF-β1)+ FoxP3+ CD8 T-cells.
Similar to previous studies in both human and RMs, we observed increased frequencies of total FoxP3+ CD8 T-cells in untreated HIV-infected individuals (15–17, 23). Notably, the reduction in FoxP3+ CD8 T-cells following early ART initiation was reported in SIV-infected RMs (38, 39), while no studies in humans, to the best of our knowledge, have evaluated its effect on HIV-infected individuals. ECs showed lower FoxP3+ CD8 T-cell frequencies than HIV-progressors and were comparable to uninfected individuals, which contrasts with a unique report of an increase in FoxP3+ CD8 T-cells in SIV controllers Indian RMs compared to SIV progressor monkeys (18). These differences could be associated with increased viral fitness and VL and faster disease progression in the Indian RM model (61). In our study, the EC group is significantly older and with a longer duration of the infection, which can also impact our observations. In line with previous reports, our results showed that total FoxP3+ CD8 T-cell frequencies were linked to markers of disease progression such as CD4 T-cell count, CD4/CD8 ratio, and VL (17).
Untreated HIV infection was associated with an early increase in FoxP3+ CD8 T-cell immune activation (HLA-DR+/CD38+) and senescence (CD28-CD57+) that remained elevated despite early ART initiation. The maintenance of activated FoxP3+ CD8 T-cells following early ART is significantly important since activated CD8 T-cells have higher proliferation (60), and HLA-DR+ CD8 T-cells are highly immunosuppressive comparable to CD4 Tregs (62). Even in the absence of viremia under successful ART, immune activation persists and promotes immunosenescence (63), which could explain higher levels of immunosenescent FoxP3+ CD8 T-cells regardless of early ART initiation. The increase in immunosenescent FoxP3+ CD8 T-cells in ECs compared to uninfected individuals, while having similar levels of immune activation, could be related to the age of these individuals since a positive correlation between age and CD28-CD57+ CD8 T-cells was only observed in ECs (data not shown) (64).
A distinctive differentiation pattern of FoxP3+ CD8 T-cells was observed in untreated HIV infection, characterized by an increase in CM, EM, and TD FoxP3+ CD8 T-cells that, excepting for EM FoxP3+ CD8 T-cells, were normalized by early ART, indicating an increased differentiation of FoxP3+ CD8 T-cells in HIV infection. The increase in total FoxP3+ CD8 T-cells and their differentiation could be related to the expansion of antigen-experienced FoxP3+ CD8 T-cells or conversion of antigen-primmed FoxP3-CD8 T-cells into FoxP3+ CD8 T-cells by TGF-β1 (65). The persistence of higher frequencies of EM FoxP3+ CD8 T-cells despite early ART is particularly important. In fact, EM T-cells show an increased ability to localize within tissues and migrate into non-lymphoid tissues in response to infection or inflammation (66, 67), suggesting a higher FoxP3+ CD8 T-cell migratory potential towards inflammatory sites and the gut. In this regard, we observed increased frequencies of FoxP3+ CD8 T-cells expressing migration markers to inflammatory sites and the gut and the persistence of CCR9/Integrin β7 FoxP3+ CD8 T-cells despite early ART. Moreover, the increase in highly differentiated FoxP3+CD8 T-cells is in line with the increase in CCR5+ FoxP3+ CD8 T-cells since CCR5 expression is associated with an increase in CD8 functions and differentiation (49, 68, 69). We also observed increased CCR4+ and CXCR3+ FoxP3+ CD8 T-cells in untreated HIV infection, which was restored by early ART initiation. Importantly, CCR4 expression is associated with higher CD4 Tregs inhibitory capacity and could have similar functions in FoxP3+ CD8 T-cells (70), while anti-CCR4 treatment decreases CD8 T-cell immune responses (71). On the other hand, CXCR3+ CD8 T-cells are well-known IL-10 producers immunosuppressor cells (72), and CXCR3 expression is a reliable marker for EM CD8 T-cells immune responses (73). In addition, CXCR3 expression regulates CD8 T-cells differentiation in acute and chronic viral infections (74). FoxP3+ CD8 T-cells could co-localize with CD4 T-cells expressing similar chemokine receptors and further inhibit their proliferation and anti-HIV-specific response. Importantly, CCR5+ and CCR6+ CD4 T-cells are highly susceptible to HIV infection (75–77). Thus, FoxP3+ CD8 T-cell colocalization with CD4 T-cells mediated by CCR5 and CCR6-dependent recruitment could contribute to poor viral control and disease progression. Moreover, a model of colocalization between HIV-specific CD8 and CD4 T-cells in the gut pointed to integrin β7 rather than CCR6 as the mediator of this migration (53).
An increase in various immunosuppressive subsets of FoxP3+ CD8 T-cells, including CTLA-4+, PD-1+, CD39+, LAP(TGF-β1)+, and CD39+LAP(TGF-β1)+ was observed in untreated HIV infection, whereas early ART initiation was unable to normalize levels of PD-1+, and did not affect CD39+ and LAP(TGF-β1)+FoxP3+ CD8 T-cells. Similarly, in the same study cohort, we also observed that early ART initiation failed to normalize PD-1+ and CD39+ CD4 Tregs (40). However, we recently reported that very early ART initiation at four days post-SIV infection of RMs reduced the frequencies of CD39+ FoxP3+ CD8 T-cells (39). Furthermore, longer ART treatment correlated negatively with CD39+ FoxP3+ CD8 T-cell frequencies, which could indicate that earlier ART initiation and longer treatment contribute to better control of their expansion. Despite early ART initiation, the persistence of PD-1+ and CD39+ FoxP3+ CD8 T-cells could contribute to immune dysfunction and disease progression. Indeed, PD-1/PD-1L contributes to FoxP3+ CD8 T-cells immunosuppression by increasing FoxP3+ CD8 T-cells proliferation/differentiation and inducing apoptosis in effector cells (21, 62). Importantly, PD-1/PD-1L interaction induces FoxP3 expression and promotes CD4 Tregs expansion (78–80). Thus, it is logical to think that a similar process might occur in CD8 T-cells promoting FoxP3 stabilization and FoxP3+ CD8 T-cells expansion. Furthermore, the increase in LAP(TGF-β1)+ FoxP3+ CD8 T-cells during untreated infection is supported by a report of a positive correlation between TGF-β1 production and FoxP3+ CD8 T-cells frequencies in non-pathogenic SIV infection in African green monkeys (23). Moreover, downstream genes of the TGF-β1 pathway are upregulated as early as one day after SIV infection in RMs (81) and HIV-infected individuals (82). FoxP3+ CD8 T-cells in ECs expressed similar CTLA-4, PD-1, CD39, and LAP(TGF-β1) levels than uninfected individuals, which could be associated with the maintenance of effector cell functions and viral control in these individuals.
Similar to our recent report on CD4 Tregs (40), we observed increased CCR9+ and integrin β7+ FoxP3+ CD8 T-cells, along with CD39+ and LAP(TGF-β1)+ FoxP3+ CD8 T-cells during untreated HIV infection that persisted regardless of early ART initiation. These findings are particularly significant, suggesting a potential migration of FoxP3+ CD8 T-cells with known immunosuppressive potential towards the gut despite ART. Interestingly, CD4 Tregs can promote the proliferation of FoxP3+ CD8 T-cells and vice versa. Indeed, each cell type’s IL-10 and TGF-β1 may contribute to FoxP3 expression and differentiation of the other subset (12, 29). Moreover, both CD4+ and CD8+FoxP3+ T-cells have previously been shown to work together in animal models, where the participation of both Treg subsets is significantly higher in combined transfers than in independent transfers (13, 83, 84). The migration of highly immunosuppressive CD39+ and LAP(TGF-β1)+ FoxP3+ CD8 T-cells to the gut could inhibit specific antiviral responses while promoting immune dysfunction and tissue fibrosis (16, 34). Notably, functional interplays between CD39 and TGF-β1 are also known. Indeed, TGF-β1 production, tissue remodeling, and fibrosis are promoted by CD39 enzymatic activity and adenosine production (85–87), whereas TGF-β1 signaling stimulates CD39 expression and activity (88–91). Moreover, an increase in TGF-β1 production and activity by the adenosine pathway may also stimulate FoxP3+ Tregs expansion (92, 93). Interestingly, TGF-β1 upregulates CTLA-4 and PD-1 expression (94), and we observed an increase in the expression of both markers. This indicates that in addition to promoting fibrosis and inducing CD39 and FoxP3 expression, TGF-β1 can also contribute to immunosuppression by inducing immune checkpoints PD-1 and CTLA-4. Notably, total CCR9+ FoxP3+ CD8 T-cells and CD39+/LAP(TGF-β1)+ FoxP3+ CD8 T-cells expressing CCR9 remained elevated despite early ART initiation, but their frequencies were negatively correlated with the duration of ART, suggesting that longer ART duration rather than earlier interventions could decrease their frequencies.
Finally, we observed an increase in both CD28-PD-1+ and CD28-CD39+ CD8 T-cells - two subsets with immunosuppressive functions regardless of FoxP3 expression - which was not restored following early ART initiation. The increase in CD28-PD-1+ (15, 95) and CD28-CD39+ (28) CD8 T-cells in untreated HIV-infected individuals correspond with previous reports. CD28- CD8 T-cells are known to induce tolerogenic dendritic cells and secretion of inhibitory cytokines such as IL-10 and TGF-β1 (96). CD28-PD-1+ phenotype is associated with immune exhaustion, poor anti-HIV specific response, and disease progression (97, 98). Thus, increased CD28-PD-1+ CD8 T-cells during untreated HIV infection and their persistence regardless of ART indicates exhaustion and potentially dysfunctionality of CD8 T-cells despite early ART. Moreover, CD28-CD39+ CD8 T-cells could contribute to immune dysfunction and disease progression through similar mechanisms than FoxP3+CD39+ CD8 T-cells.
Our study had some limitations which deserve to be discussed, including the relatively small sample size. Nevertheless, our findings were consistent with previous findings using a similar sample size and had a high biological plausibility (40, 99, 100). We recognize that variables such as gender, age, duration of infection, and timing of ART initiation may influence our findings. In this regard, the expression of FoxP3 and other Treg markers can be influenced by sex hormones and gender (101, 102). Similarly, the frequencies and functions of FoxP3+ CD8 T-cells and the expression of immunosuppressive markers by these cells differ in older individuals (103, 104). We mainly recruited male participants in our analysis since they constitute the majority of the participants in the Montreal primary HIV infection (acute) cohort. Additionally, we did not provide functional assays to assess FoxP3+ CD8 T-cells’ inhibitory capacity since we had limited access to these specimens and the fact that FACS-sorting of FoxP3+ CD8 T-cells requires the permeabilization and fixation of the cells which are not usable for in vitro functional assays. Ultimately, while we used well-established markers of T-cell migration to the gut, all analyses were performed in peripheral blood as an indirect indication of FoxP3+ CD8 T-cell migration towards this compartment.
In summary, for the first time, we spotlight various subsets of FoxP3+ CD8 T-cells that might be critical in HIV disease progression. We showed that early ART initiation did not normalize the frequency of immunosuppressive and pro-fibrogenic FoxP3+ CD8 T-cells and their potential migration to the gut. The latter can contribute to immune dysfunction, gut fibrosis, and HIV disease progression, suggesting that other therapies combined with early ART initiation are needed to reduce FoxP3+ CD8 T-cells immunosuppressive subsets.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by the ethical review board of the Université du Québec à Montréal (UQAM) gave their approval to this study (#2014-452), which followed the Declaration of Helsinki. All study participants signed a written informed consent form before blood collection. The patients/participants provided their written informed consent to participate in this study.
Author contributions
M-AJ designed the study. AY and TS performed the experiments. J-PR, CT, and CC provided access to specimens and clinical data. AY, TS, MD, CC, and M-AJ analysed, discussed, and interpreted results throughout the study. AY and M-AJ wrote the paper. All authors contributed to the refinement of the study and reviewed and approved the final version of manuscript.
Funding
This study was funded by the Canadian Institutes of Health Research (CIHR, grant MOP 142294) and in part by the AIDS and Infectious Diseases Network of the Réseau SIDA et maladies infectieuses du Fonds de recherche du Québec-Santé (FRQ-S) to M-AJ. AY is the recipient of an FRQ-S doctorate fellowship. J-PR is the Louis Lowenstein Chair in Hematology and Oncology at McGill University. CT is the Pfizer Chair in Clinical and Translational HIV Research. MD and CC are recipients of FRQ-S Junior 2 Clinician-Researcher career awards. M-AJ holds the tier 2 CIHR Canada Research Chair in Immuno-Virology. The funding institutions had no involvement in the data collection, analysis, or interpretation.
Acknowledgments
First and foremost, the authors like to express their gratitude to all study participants for their time and commitment to this research. Individuals in acute infection were screened, recruited, and followed up at the following Montreal-based clinics: l’Actuel Medical Clinic, Quartier Latin Medical Clinic, UHRESS CHUM Hôtel-Dieu, and Notre-Dame, and MUHC Chest Institute. We gratefully acknowledge their assistance. Mr. Mario Legault provided administrative help, Ms. Angie Massicotte provided assistance and coordination, and Ms. Stephanie Matte assisted us with the Canadian HIV Slow Progressor cohort.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2022.962912/full#supplementary-material
References
- 1. Gershon RK, Kondo K. Cell interactions in the induction of tolerance: the role of thymic lymphocytes. Immunology (1970) 18(5):723–37. [PMC free article] [PubMed] [Google Scholar]
- 2. Zhang S, Wu M, Wang F. Immune regulation by CD8(+) treg cells: novel possibilities for anticancer immunotherapy. Cell Mol Immunol (2018) 15(9):805–7. doi: 10.1038/cmi.2018.170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Yu Y, Ma X, Gong R, Zhu J, Wei L, Yao J. Recent advances in CD8(+) regulatory T cell research. Oncol Lett (2018) 15(6):8187–94. doi: 10.3892/ol.2018.8378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Vieyra-Lobato MR, Vela-Ojeda J, Montiel-Cervantes L, Lopez-Santiago R, Moreno-Lafont MC. Description of CD8(+) regulatory T lymphocytes and their specific intervention in graft-versus-Host and infectious diseases, autoimmunity, and cancer. J Immunol Res (2018) 2018:3758713. doi: 10.1155/2018/3758713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bezie S, Anegon I, Guillonneau C. Advances on CD8+ treg cells and their potential in transplantation. Transplantation (2018) 102(9):1467–78. doi: 10.1097/TP.0000000000002258 [DOI] [PubMed] [Google Scholar]
- 6. Flippe L, Bezie S, Anegon I, Guillonneau C. Future prospects for CD8(+) regulatory T cells in immune tolerance. Immunol Rev (2019) 292(1):209–24. doi: 10.1111/imr.12812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Niederlova V, Tsyklauri O, Chadimova T, Stepanek O. CD8(+) tregs revisited: A heterogeneous population with different phenotypes and properties. Eur J Immunol (2021) 51(3):512–30. doi: 10.1002/eji.202048614 [DOI] [PubMed] [Google Scholar]
- 8. Mishra S, Srinivasan S, Ma C, Zhang N. CD8(+) regulatory T cell - a mystery to be revealed. Front Immunol (2021) 12:708874. doi: 10.3389/fimmu.2021.708874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tang X, Kumar V. Advances in the study of CD8+ regulatory T cells. Crit Rev Immunol (2019) 39(6):409–21. doi: 10.1615/CritRevImmunol.2020033260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lin W, Haribhai D, Relland LM, Truong N, Carlson MR, Williams CB, et al. Regulatory T cell development in the absence of functional Foxp3. Nat Immunol (2007) 8(4):359–68. doi: 10.1038/ni1445 [DOI] [PubMed] [Google Scholar]
- 11. Churlaud G, Pitoiset F, Jebbawi F, Lorenzon R, Bellier B, Rosenzwajg M, et al. Human and mouse CD8(+)CD25(+)FOXP3(+) regulatory T cells at steady state and during interleukin-2 therapy. Front Immunol (2015) 6:171. doi: 10.3389/fimmu.2015.00171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Iamsawat S, Daenthanasanmak A, Voss JH, Nguyen H, Bastian D, Liu C, et al. Stabilization of Foxp3 by targeting JAK2 enhances efficacy of CD8 induced regulatory T cells in the prevention of graft-versus-Host disease. J Immunol (2018) 201(9):2812–23. doi: 10.4049/jimmunol.1800793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bezie S, Picarda E, Ossart J, Tesson L, Usal C, Renaudin K, et al. IL-34 is a treg-specific cytokine and mediates transplant tolerance. J Clin Invest (2015) 125(10):3952–64. doi: 10.1172/JCI81227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lim A, Tan D, Price P, Kamarulzaman A, Tan HY, James I, et al. Proportions of circulating T cells with a regulatory cell phenotype increase with HIV-associated immune activation and remain high on antiretroviral therapy. AIDS (2007) 21(12):1525–34. doi: 10.1097/QAD.0b013e32825eab8b [DOI] [PubMed] [Google Scholar]
- 15. Lim A, French MA, Price P. CD4+ and CD8+ T cells expressing FoxP3 in HIV-infected patients are phenotypically distinct and influenced by disease severity and antiretroviral therapy. J Acquir Immune Defic Syndr (2009) 51(3):248–57. doi: 10.1097/QAI.0b013e3181a74fad [DOI] [PubMed] [Google Scholar]
- 16. Nigam P, Velu V, Kannanganat S, Chennareddi L, Kwa S, Siddiqui M, et al. Expansion of FOXP3+ CD8 T cells with suppressive potential in colorectal mucosa following a pathogenic simian immunodeficiency virus infection correlates with diminished antiviral T cell response and viral control. J Immunol (2010) 184(4):1690–701. doi: 10.4049/jimmunol.0902955 [DOI] [PubMed] [Google Scholar]
- 17. Karlsson I, Malleret B, Brochard P, Delache B, Calvo J, Le Grand R, et al. FoxP3+ CD25+ CD8+ T-cell induction during primary simian immunodeficiency virus infection in cynomolgus macaques correlates with low CD4+ T-cell activation and high viral load. J Virol (2007) 81(24):13444–55. doi: 10.1128/JVI.01466-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Khowawisetsut L, Pattanapanyasat K, Onlamoon N, Mayne AE, Little DM, Villinger F, et al. Relationships between IL-17(+) subsets, tregs and pDCs that distinguish among SIV infected elite controllers, low, medium and high viral load rhesus macaques. PloS One (2013) 8(4):e61264. doi: 10.1371/journal.pone.0061264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lu W, Chen S, Lai C, Guo W, Fu L, Andrieu JM. Induction of CD8+ regulatory T cells protects macaques against SIV challenge. Cell Rep (2012) 2(6):1736–46. doi: 10.1016/j.celrep.2012.11.016 [DOI] [PubMed] [Google Scholar]
- 20. Andrieu JM, Chen S, Lai C, Guo W, Lu W. Mucosal SIV vaccines comprising inactivated virus particles and bacterial adjuvants induce CD8(+) T-regulatory cells that suppress SIV-positive CD4(+) T-cell activation and prevent SIV infection in the macaque model. Front Immunol (2014) 5:297. doi: 10.3389/fimmu.2014.00297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Horwitz DA, Pan S, Ou JN, Wang J, Chen M, Gray JD, et al. Therapeutic polyclonal human CD8+ CD25+ Fox3+ TNFR2+ PD-L1+ regulatory cells induced ex-vivo. Clin Immunol (2013) 149(3):450–63. doi: 10.1016/j.clim.2013.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Boor PP, Metselaar HJ, Jonge S, Mancham S, van der Laan LJ, Kwekkeboom J. Human plasmacytoid dendritic cells induce CD8(+) LAG-3(+) Foxp3(+) CTLA-4(+) regulatory T cells that suppress allo-reactive memory T cells. Eur J Immunol (2011) 41(6):1663–74. doi: 10.1002/eji.201041229 [DOI] [PubMed] [Google Scholar]
- 23. Kornfeld C, Ploquin MJ, Pandrea I, Faye A, Onanga R, Apetrei C, et al. Antiinflammatory profiles during primary SIV infection in African green monkeys are associated with protection against AIDS. J Clin Invest (2005) 115(4):1082–91. doi: 10.1172/JCI23006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gubser C, Chiu C, Lewin SR, Rasmussen TA. Immune checkpoint blockade in HIV. EBioMedicine (2022) 76:103840. doi: 10.1016/j.ebiom.2022.103840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jenabian MA, Ancuta P, Gilmore N, Routy JP. Regulatory T cells in HIV infection: can immunotherapy regulate the regulator? Clin Dev Immunol (2012) 2012:908314. doi: 10.1155/2012/908314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Deaglio S, Dwyer KM, Gao W, Friedman D, Usheva A, Erat A, et al. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J Exp Med (2007) 204(6):1257–65. doi: 10.1084/jem.20062512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jenabian MA, Seddiki N, Yatim A, Carriere M, Hulin A, Younas M, et al. Regulatory T cells negatively affect IL-2 production of effector T cells through CD39/adenosine pathway in HIV infection. PloS Pathog (2013) 9(4):e1003319. doi: 10.1371/journal.ppat.1003319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fenoglio D, Dentone C, Signori A, Di Biagio A, Parodi A, Kalli F, et al. CD8(+)CD28(-)CD127(lo)CD39(+) regulatory T-cell expansion: A new possible pathogenic mechanism for HIV infection? J Allergy Clin Immunol (2018) 141(6):2220–33.e4. doi: 10.1016/j.jaci.2017.08.021 [DOI] [PubMed] [Google Scholar]
- 29. Lerret NM, Houlihan JL, Kheradmand T, Pothoven KL, Zhang ZJ, Luo X. Donor-specific CD8+ Foxp3+ T cells protect skin allografts and facilitate induction of conventional CD4+ Foxp3+ regulatory T cells. Am J Transplant (2012) 12(9):2335–47. doi: 10.1111/j.1600-6143.2012.04120.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Li MO, Wan YY, Sanjabi S, Robertson AK, Flavell RA. Transforming growth factor-beta regulation of immune responses. Annu Rev Immunol (2006) 24:99–146. doi: 10.1146/annurev.immunol.24.021605.090737 [DOI] [PubMed] [Google Scholar]
- 31. Kim KK, Sheppard D, Chapman HA. TGF-beta1 signaling and tissue fibrosis. Cold Spring Harb Perspect Biol (2018) 10(4):a022293. doi: 10.1101/cshperspect.a022293"10.1101/cshperspect.a02229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Estes JD, Wietgrefe S, Schacker T, Southern P, Beilman G, Reilly C, et al. Simian immunodeficiency virus-induced lymphatic tissue fibrosis is mediated by transforming growth factor beta 1-positive regulatory T cells and begins in early infection. J Infect Dis (2007) 195(4):551–61. doi: 10.1086/510852 [DOI] [PubMed] [Google Scholar]
- 33. Zeng M, Smith AJ, Wietgrefe SW, Southern PJ, Schacker TW, Reilly CS, et al. Cumulative mechanisms of lymphoid tissue fibrosis and T cell depletion in HIV-1 and SIV infections. J Clin Invest (2011) 121(3):998–1008. doi: 10.1172/JCI45157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Huang L, Deng J, Xu W, Wang H, Shi L, Wu F, et al. CD8+ T cells with high TGFbeta1 expression cause lymph node fibrosis following HIV infection. Mol Med Rep (2018) 18(1):77–86. doi: 10.3892/mmr.2018.8964"10.3892/mmr.2018.8964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sanchez JL, Hunt PW, Reilly CS, Hatano H, Beilman GJ, Khoruts A, et al. Lymphoid fibrosis occurs in long-term nonprogressors and persists with antiretroviral therapy but may be reversible with curative interventions. J Infect Dis (2015) 211(7):1068–75. doi: 10.1093/infdis/jiu586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rajasuriar R, Wright E, Lewin SR. Impact of antiretroviral therapy (ART) timing on chronic immune activation/inflammation and end-organ damage. Curr Opin HIV AIDS (2015) 10(1):35–42. doi: 10.1097/COH.0000000000000118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Maartens G, Celum C, Lewin SR. HIV Infection: epidemiology, pathogenesis, treatment, and prevention. Lancet (2014) 384(9939):258–71. doi: 10.1016/S0140-6736(14)60164-1 [DOI] [PubMed] [Google Scholar]
- 38. George J, Cofano EB, Lybarger E, Louder M, Lafont BA, Mascola JR, et al. Early short-term antiretroviral therapy is associated with a reduced prevalence of CD8(+)FoxP3(+) T cells in simian immunodeficiency virus-infected controller rhesus macaques. AIDS Res Hum Retroviruses (2011) 27(7):763–75. doi: 10.1089/aid.2010.0251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yero A, Farnos O, Clain J, Zghidi-Abouzid O, Rabezanahary H, Racine G, et al. Impact of early ARV initiation on relative proportions of effector and regulatory CD8 T cell in mesenteric lymph nodes and peripheral blood during acute SIV infection of rhesus macaques. J Virol (2022) 96:e0025522. doi: 10.1128/jvi.00255-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Yero A, Shi T, Farnos O, Routy JP, Tremblay C, Durand M, et al. Dynamics and epigenetic signature of regulatory T-cells following antiretroviral therapy initiation in acute HIV infection. EBioMedicine (2021) 71:103570. doi: 10.1016/j.ebiom.2021.103570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Carriere M, Lacabaratz C, Kok A, Benne C, Jenabian MA, Casartelli N, et al. HIV "elite controllers" are characterized by a high frequency of memory CD8+ CD73+ T cells involved in the antigen-specific CD8+ T-cell response. J Infect Dis (2014) 209(9):1321–30. doi: 10.1093/infdis/jit643 [DOI] [PubMed] [Google Scholar]
- 42. Yoshie O. CCR4 as a therapeutic target for cancer immunotherapy. Cancers (Basel) (2021) 13(21):5542. doi: 10.3390/cancers13215542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Casciano F, Diani M, Altomare A, Granucci F, Secchiero P, Banfi G, et al. CCR4(+) skin-tropic phenotype as a feature of central memory CD8(+) T cells in healthy subjects and psoriasis patients. Front Immunol (2020) 11:529. doi: 10.3389/fimmu.2020.00529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Spoerl S, Kremer AN, Aigner M, Eisenhauer N, Koch P, Meretuk L, et al. Upregulation of CCR4 in activated CD8(+) T cells indicates enhanced lung homing in patients with severe acute SARS-CoV-2 infection. Eur J Immunol (2021) 51(6):1436–48. doi: 10.1002/eji.202049135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Huser N, Tertilt C, Gerauer K, Maier S, Traeger T, Assfalg V, et al. CCR4-deficient mice show prolonged graft survival in a chronic cardiac transplant rejection model. Eur J Immunol (2005) 35(1):128–38. doi: 10.1002/eji.200324745 [DOI] [PubMed] [Google Scholar]
- 46. Vangelista L, Vento S. The expanding therapeutic perspective of CCR5 blockade. Front Immunol (2017) 8:1981. doi: 10.3389/fimmu.2017.01981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Jiao X, Nawab O, Patel T, Kossenkov AV, Halama N, Jaeger D, et al. Recent advances targeting CCR5 for cancer and its role in immuno-oncology. Cancer Res (2019) 79(19):4801–7. doi: 10.1158/0008-5472.CAN-19-1167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lind L, Svensson A, Thorn K, Krzyzowska M, Eriksson K. CD8(+) T cells in the central nervous system of mice with herpes simplex infection are highly activated and express high levels of CCR5 and CXCR3. J Neurovirol (2021) 27(1):145–53. doi: 10.1007/s13365-020-00940-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wang X, Russell-Lodrigue KE, Ratterree MS, Veazey RS, Xu H. Chemokine receptor CCR5 correlates with functional CD8(+) T cells in SIV-infected macaques and the potential effects of maraviroc on T-cell activation. FASEB J (2019) 33(8):8905–12. doi: 10.1096/fj.201802703R [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Karin N. CXCR3 ligands in cancer and autoimmunity, chemoattraction of effector T cells, and beyond. Front Immunol (2020) 11:976. doi: 10.3389/fimmu.2020.00976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kuo PT, Zeng Z, Salim N, Mattarollo S, Wells JW, Leggatt GR. The role of CXCR3 and its chemokine ligands in skin disease and cancer. Front Med (Lausanne) (2018) 5:271. doi: 10.3389/fmed.2018.00271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kurachi M, Kurachi J, Suenaga F, Tsukui T, Abe J, Ueha S, et al. Chemokine receptor CXCR3 facilitates CD8(+) T cell differentiation into short-lived effector cells leading to memory degeneration. J Exp Med (2011) 208(8):1605–20. doi: 10.1084/jem.20102101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Wacleche VS, Chomont N, Gosselin A, Monteiro P, Goupil M, Kared H, et al. The colocalization potential of HIV-specific CD8+ and CD4+ T-cells is mediated by integrin beta7 but not CCR6 and regulated by retinoic acid. PloS One (2012) 7(3):e32964. doi: 10.1371/journal.pone.0032964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kondo T, Takata H, Takiguchi M. Functional expression of chemokine receptor CCR6 on human effector memory CD8+ T cells. Eur J Immunol (2007) 37(1):54–65. doi: 10.1002/eji.200636251 [DOI] [PubMed] [Google Scholar]
- 55. Trivett MT, Burke JD, Deleage C, Coren LV, Hill BJ, Jain S, et al. Preferential small intestine homing and persistence of CD8 T cells in rhesus macaques achieved by molecularly engineered expression of CCR9 and reduced ex vivo manipulation. J Virol (2019) 93(21):e00896–19. doi: 10.1128/JVI.00896-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Wermers JD, McNamee EN, Wurbel MA, Jedlicka P, Rivera-Nieves J. The chemokine receptor CCR9 is required for the T-cell-mediated regulation of chronic ileitis in mice. Gastroenterology (2011) 140(5):1526–35.e3. doi: 10.1053/j.gastro.2011.01.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Eksteen B, Mora JR, Haughton EL, Henderson NC, Lee-Turner L, Villablanca EJ, et al. Gut homing receptors on CD8 T cells are retinoic acid dependent and not maintained by liver dendritic or stellate cells. Gastroenterology (2009) 137(1):320–9. doi: 10.1053/j.gastro.2009.02.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Chen X, Liu Q, Xiang AP. CD8+CD28- T cells: not only age-related cells but a subset of regulatory T cells. Cell Mol Immunol (2018) 15(8):734–6. doi: 10.1038/cmi.2017.153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Perdomo-Celis F, Taborda NA, Rugeles MT. CD8(+) T-cell response to HIV infection in the era of antiretroviral therapy. Front Immunol (2019) 10:1896. doi: 10.3389/fimmu.2019.01896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Gonzalez SM, Taborda NA, Rugeles MT. Role of different subpopulations of CD8(+) T cells during HIV exposure and infection. Front Immunol (2017) 8:936. doi: 10.3389/fimmu.2017.00936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Terrade G, Huot N, Petitdemange C, Lazzerini M, Orta Resendiz A, Jacquelin B, et al. Interests of the non-human primate models for HIV cure research. Vaccines (Basel) (2021) 9(9). doi: 10.3390/vaccines9090958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Machicote A, Belen S, Baz P, Billordo LA, Fainboim L. Human CD8(+)HLA-DR(+) regulatory T cells, similarly to classical CD4(+)Foxp3(+) cells, suppress immune responses via PD-1/PD-L1 axis. Front Immunol (2018) 9:2788. doi: 10.3389/fimmu.2018.02788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Moir S, Chun TW, Fauci AS. Pathogenic mechanisms of HIV disease. Annu Rev Pathol (2011) 6:223–48. doi: 10.1146/annurev-pathol-011110-130254 [DOI] [PubMed] [Google Scholar]
- 64. Aiello A, Farzaneh F, Candore G, Caruso C, Davinelli S, Gambino CM, et al. Immunosenescence and its hallmarks: How to oppose aging strategically? a review of potential options for therapeutic intervention. Front Immunol (2019) 10:2247. doi: 10.3389/fimmu.2019.02247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Fan H, Huo X, Sun J, Yang Y, Li X. Efficient induction and expansion of CD8+CD28+Foxp3+ regulatory T cells by TGF-beta1 and rapamycin. Blood (2013) 122(21):190–. doi: 10.1182/blood.V122.21.190.190 [DOI] [Google Scholar]
- 66. Masopust D, Vezys V, Marzo AL, Lefrancois L. Preferential localization of effector memory cells in nonlymphoid tissue. Science (2001) 291(5512):2413–7. doi: 10.1126/science.1058867 [DOI] [PubMed] [Google Scholar]
- 67. Martin MD, Badovinac VP. Defining memory CD8 T cell. Front Immunol (2018) 9:2692. doi: 10.3389/fimmu.2018.02692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Askew D, Su CA, Barkauskas DS, Dorand RD, Myers J, Liou R, et al. Transient surface CCR5 expression by naive CD8+ T cells within inflamed lymph nodes is dependent on high endothelial venule interaction and augments Th cell-dependent memory response. J Immunol (2016) 196(9):3653–64. doi: 10.4049/jimmunol.1501176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Li R, Zhang N, Tian M, Ran Z, Zhu M, Zhu H, et al. Temporary CXCR3 and CCR5 antagonism following vaccination enhances memory CD8 T cell immune responses. Mol Med (2016) 22:497–507. doi: 10.2119/molmed.2015.00218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Ferraro A, D'Alise AM, Raj T, Asinovski N, Phillips R, Ergun A, et al. Interindividual variation in human T regulatory cells. Proc Natl Acad Sci U S A (2014) 111(12):E1111–20. doi: 10.1073/pnas.1401343111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Sugiyama D, Nishikawa H, Maeda Y, Nishioka M, Tanemura A, Katayama I, et al. Anti-CCR4 mAb selectively depletes effector-type FoxP3+CD4+ regulatory T cells, evoking antitumor immune responses in humans. Proc Natl Acad Sci U S A (2013) 110(44):17945–50. doi: 10.1073/pnas.1316796110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Shi Z, Okuno Y, Rifa'i M, Endharti AT, Akane K, Isobe K-i, et al. Human CD8+CXCR3+ T cells have the same function as murine CD8+CD122+ treg. Eur J Immunol (2009) 39(8):2106–19. doi: 10.1002/eji.200939314 [DOI] [PubMed] [Google Scholar]
- 73. Groom JR, Luster AD. CXCR3 in T cell function. Exp Cell Res (2011) 317(5):620–31. doi: 10.1016/j.yexcr.2010.12.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Bangs DJ, Tsitsiklis A, Steier Z, Chan SW, Kaminski J, Streets A, et al. CXCR3 regulates stem and proliferative CD8+ T cells during chronic infection by promoting interactions with DCs in splenic bridging channels. Cell Rep (2022) 38(3):110266. doi: 10.1016/j.celrep.2021.110266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Mirza MU, Saadabadi A, Vanmeert M, Salo-Ahen OMH, Abdullah I, Claes S, et al. Discovery of HIV entry inhibitors via a hybrid CXCR4 and CCR5 receptor pharmacophore-based virtual screening approach. Eur J Pharm Sci (2020) 155:105537. doi: 10.1016/j.ejps.2020.105537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Islam S, Shimizu N, Hoque SA, Jinno-Oue A, Tanaka A, Hoshino H. CCR6 functions as a new coreceptor for limited primary human and simian immunodeficiency viruses. PloS One (2013) 8(8):e73116. doi: 10.1371/journal.pone.0073116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Planas D, Zhang Y, Monteiro P, Goulet JP, Gosselin A, Grandvaux N, et al. HIV-1 selectively targets gut-homing CCR6+CD4+ T cells via mTOR-dependent mechanisms. JCI Insight (2017) 2(15). doi: 10.1172/jci.insight.93230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Fanelli G, Romano M, Nova-Lamperti E, Werner Sunderland M, Nerviani A, Scotta C, et al. PD-L1 signaling on human memory CD4+ T cells induces a regulatory phenotype. PloS Biol (2021) 19(4):e3001199. doi: 10.1371/journal.pbio.3001199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Chen X, Fosco D, Kline DE, Meng L, Nishi S, Savage PA, et al. PD-1 regulates extrathymic regulatory T-cell differentiation. Eur J Immunol (2014) 44(9):2603–16. doi: 10.1002/eji.201344423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Dong Y, Han Y, Huang Y, Jiang S, Huang Z, Chen R, et al. PD-L1 is expressed and promotes the expansion of regulatory T cells in acute myeloid leukemia. Front Immunol (2020) 11:1710. doi: 10.3389/fimmu.2020.01710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Barouch DH, Ghneim K, Bosche WJ, Li Y, Berkemeier B, Hull M, et al. Rapid inflammasome activation following mucosal SIV infection of rhesus monkeys. Cell (2016) 165(3):656–67. doi: 10.1016/j.cell.2016.03.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Dickinson M, Kliszczak AE, Giannoulatou E, Peppa D, Pellegrino P, Williams I, et al. Dynamics of transforming growth factor (TGF)-beta superfamily cytokine induction during HIV-1 infection are distinct from other innate cytokines. Front Immunol (2020) 11:596841. doi: 10.3389/fimmu.2020.596841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Picarda E, Bezie S, Boucault L, Autrusseau E, Kilens S, Meistermann D, et al. Transient antibody targeting of CD45RC induces transplant tolerance and potent antigen-specific regulatory T cells. JCI Insight (2017) 2(3):e90088. doi: 10.1172/jci.insight.90088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Heinrichs J, Li J, Nguyen H, Wu Y, Bastian D, Daethanasanmak A, et al. CD8(+) tregs promote GVHD prevention and overcome the impaired GVL effect mediated by CD4(+) tregs in mice. Oncoimmunology (2016) 5(6):e1146842. doi: 10.1080/2162402X.2016.1146842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Wen J, Jiang X, Dai Y, Zhang Y, Tang Y, Sun H, et al. Increased adenosine contributes to penile fibrosis, a dangerous feature of priapism, via A2B adenosine receptor signaling. FASEB J (2010) 24(3):740–9. doi: 10.1096/fj.09-144147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Luo F, Le NB, Mills T, Chen NY, Karmouty-Quintana H, Molina JG, et al. Extracellular adenosine levels are associated with the progression and exacerbation of pulmonary fibrosis. FASEB J (2016) 30(2):874–83. doi: 10.1096/fj.15-274845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Kunzli BM, Nuhn P, Enjyoji K, Banz Y, Smith RN, Csizmadia E, et al. Disordered pancreatic inflammatory responses and inhibition of fibrosis in CD39-null mice. Gastroenterology (2008) 134(1):292–305. doi: 10.1053/j.gastro.2007.10.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Peres RS, Donate PB, Talbot J, Cecilio NT, Lobo PR, Machado CC, et al. TGF-beta signalling defect is linked to low CD39 expression on regulatory T cells and methotrexate resistance in rheumatoid arthritis. J Autoimmun (2018) 90:49–58. doi: 10.1016/j.jaut.2018.01.004 [DOI] [PubMed] [Google Scholar]
- 89. Ye ZJ, Zhou Q, Zhang JC, Li X, Wu C, Qin SM, et al. CD39+ regulatory T cells suppress generation and differentiation of Th17 cells in human malignant pleural effusion via a LAP-dependent mechanism. Respir Res (2011) 12(1):77. doi: 10.1186/1465-9921-12-77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Regateiro FS, Howie D, Nolan KF, Agorogiannis EI, Greaves DR, Cobbold SP, et al. Generation of anti-inflammatory adenosine by leukocytes is regulated by TGF-beta. Eur J Immunol (2011) 41(10):2955–65. doi: 10.1002/eji.201141512 [DOI] [PubMed] [Google Scholar]
- 91. Gerner MC, Ziegler LS, Schmidt RLJ, Krenn M, Zimprich F, Uyanik-Unal K, et al. The TGF-b/SOX4 axis and ROS-driven autophagy co-mediate CD39 expression in regulatory T-cells. FASEB J (2020) 34(6):8367–84. doi: 10.1096/fj.201902664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Zarek PE, Huang CT, Lutz ER, Kowalski J, Horton MR, Linden J, et al. A2A receptor signaling promotes peripheral tolerance by inducing T-cell anergy and the generation of adaptive regulatory T cells. Blood (2008) 111(1):251–9. doi: 10.1182/blood-2007-03-081646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Ohta A, Kini R, Ohta A, Subramanian M, Madasu M, Sitkovsky M. The development and immunosuppressive functions of CD4(+) CD25(+) FoxP3(+) regulatory T cells are under influence of the adenosine-A2A adenosine receptor pathway. Front Immunol (2012) 3(190):190. doi: 10.3389/fimmu.2012.00190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Bao S, Jiang X, Jin S, Tu P, Lu J. TGF-β1 induces immune escape by enhancing PD-1 and CTLA-4 expression on T lymphocytes in hepatocellular carcinoma. Front Oncol (2021) 11. doi: 10.3389/fonc.2021.694145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Elrefaei M, Baker CA, Jones NG, Bangsberg DR, Cao H. Presence of suppressor HIV-specific CD8+ T cells is associated with increased PD-1 expression on effector CD8+ T cells. J Immunol (2008) 180(11):7757–63. doi: 10.4049/jimmunol.180.11.7757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Vuddamalay Y, van Meerwijk JPM. CD28(-) and CD28(low)CD8(+) regulatory T cells: Of mice and men. Front Immunol (2017) 8:31–. doi: 10.3389/fimmu.2017.00031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Trautmann L, Janbazian L, Chomont N, Said EA, Gimmig S, Bessette B, et al. Upregulation of PD-1 expression on HIV-specific CD8+ T cells leads to reversible immune dysfunction. Nat Med (2006) 12(10):1198–202. doi: 10.1038/nm1482 [DOI] [PubMed] [Google Scholar]
- 98. Day CL, Kaufmann DE, Kiepiela P, Brown JA, Moodley ES, Reddy S, et al. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature (2006) 443(7109):350–4. doi: 10.1038/nature05115 [DOI] [PubMed] [Google Scholar]
- 99. Jenabian MA, El-Far M, Vyboh K, Kema I, Costiniuk CT, Thomas R, et al. Immunosuppressive tryptophan catabolism and gut mucosal dysfunction following early HIV infection. J Infect Dis (2015) 212(3):355–66. doi: 10.1093/infdis/jiv037 [DOI] [PubMed] [Google Scholar]
- 100. Jenabian MA, Patel M, Kema I, Vyboh K, Kanagaratham C, Radzioch D, et al. Soluble CD40-ligand (sCD40L, sCD154) plays an immunosuppressive role via regulatory T cell expansion in HIV infection. Clin Exp Immunol (2014) 178(1):102–11. doi: 10.1111/cei.12396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Singh RP, Bischoff DS. Sex hormones and gender influence the expression of markers of regulatory T cells in SLE patients. Front Immunol (2021) 12. doi: 10.3389/fimmu.2021.619268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Nie J, Li YY, Zheng SG, Tsun A, Li B. FOXP3(+) treg cells and gender bias in autoimmune diseases. Front Immunol (2015) 6:493–. doi: 10.3389/fimmu.2015.00493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Jagger A, Shimojima Y, Goronzy JJ, Weyand CM. Regulatory T cells and the immune aging process: a mini-review. Gerontology (2014) 60(2):130–7. doi: 10.1159/000355303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Lukas Yani S, Keller M, Melzer FL, Weinberger B, Pangrazzi L, Sopper S, et al. CD8+HLADR+ regulatory T cells change with aging: They increase in number, but lose checkpoint inhibitory molecules and suppressive function. Front Immunol (2018) 9. doi: 10.3389/fimmu.2018.01201 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.